Synthesis of Cyanocobalamin
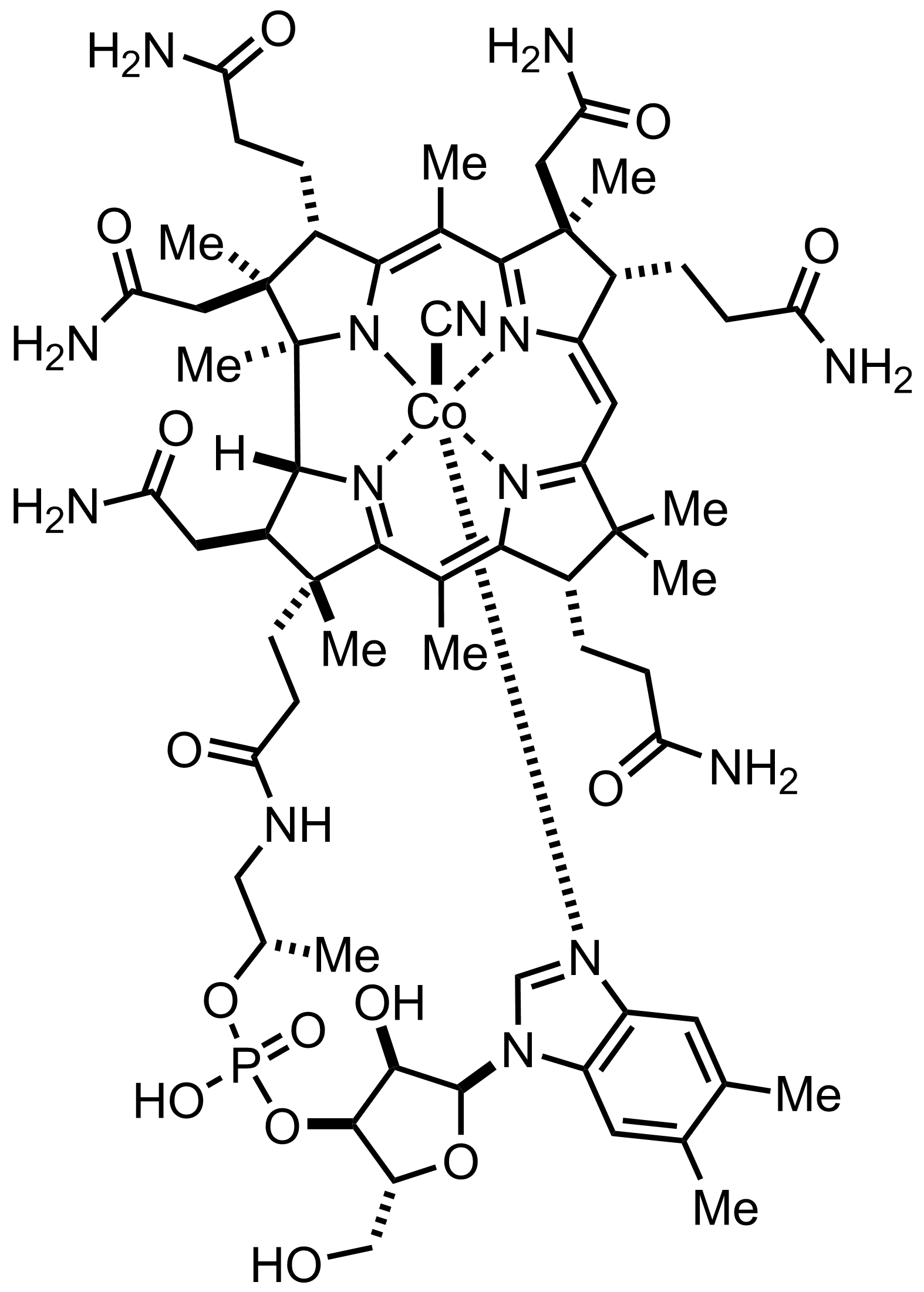
C63H89CoN14O14P
| Principal investigator | Robert B. Woodward |
|---|---|
| Publication year | 1973 |
| Synthesis type | Formal |
| Number of steps | 72 (7 parts) |
| References |
Part 1 of 7
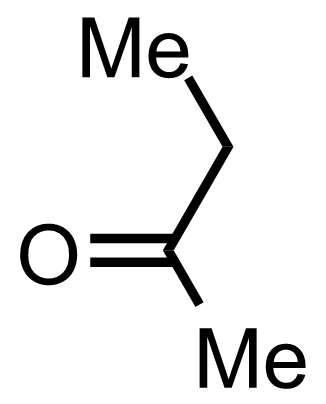 +
+
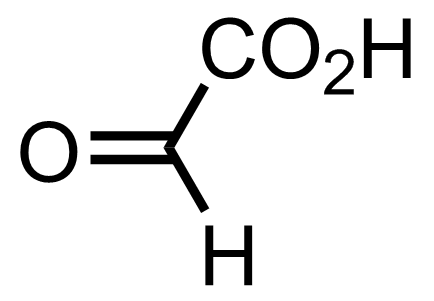
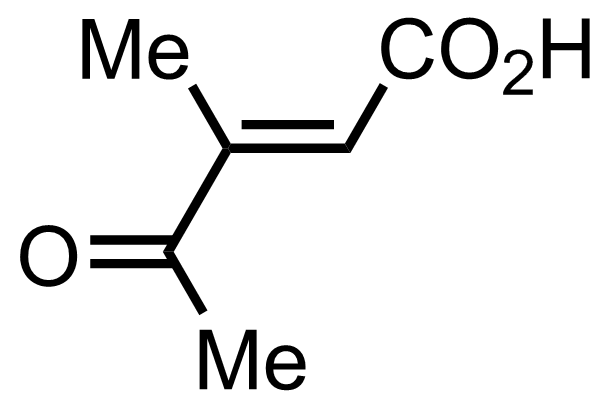 +
+
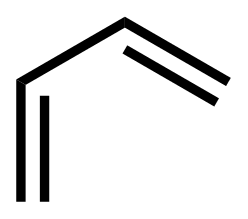
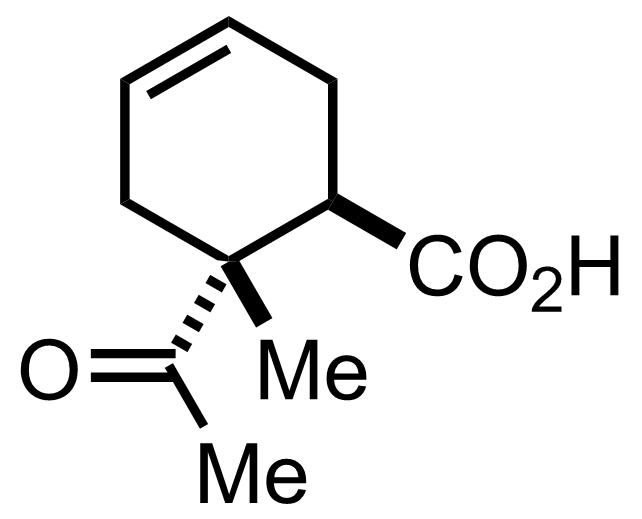
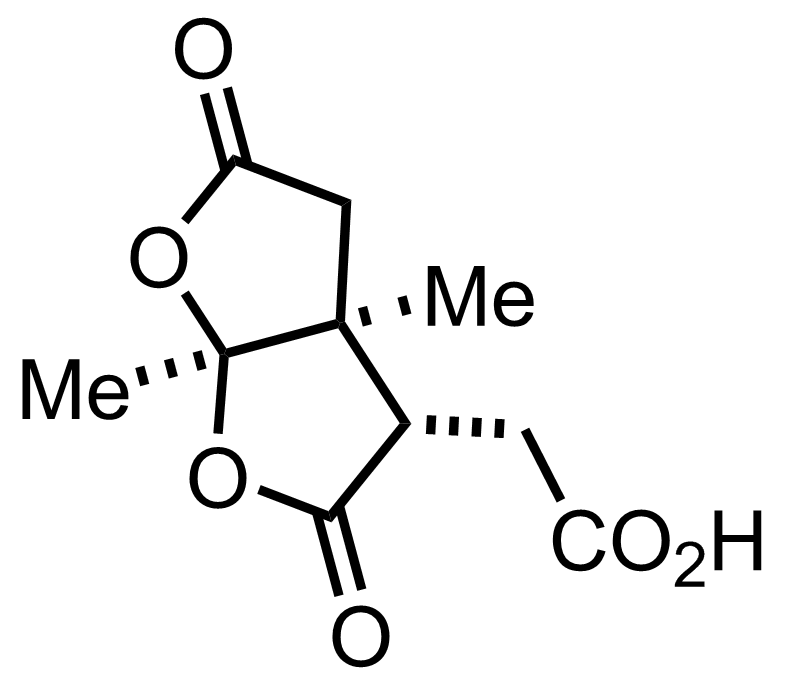
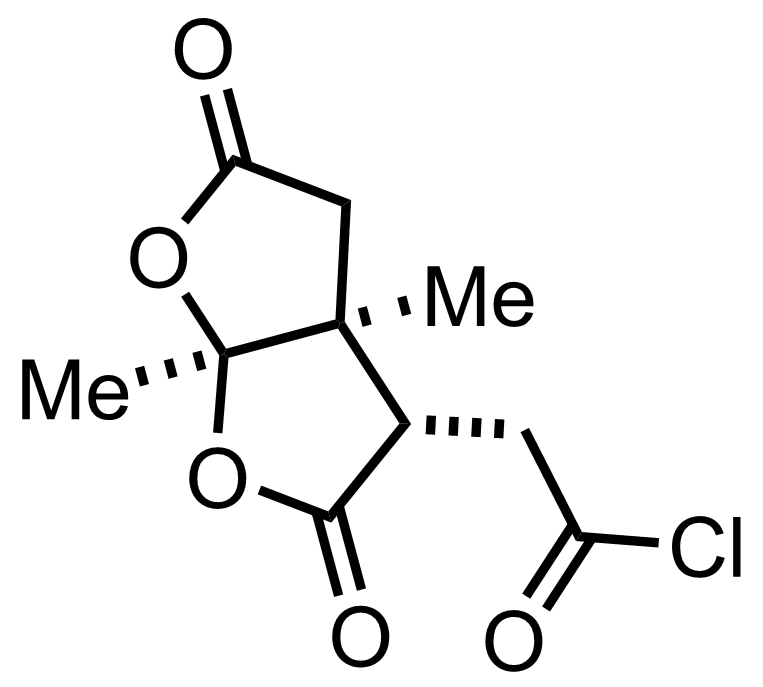
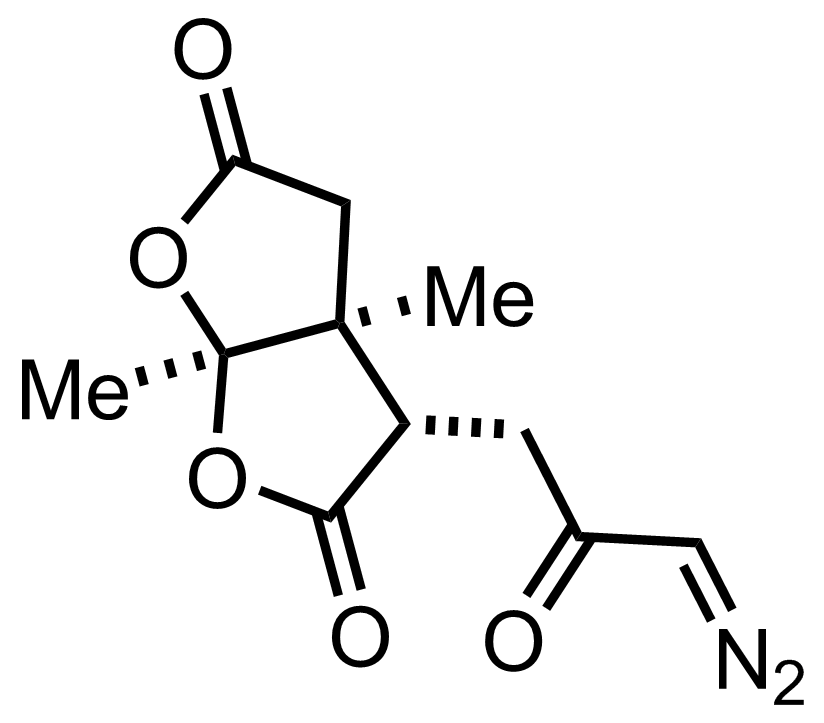
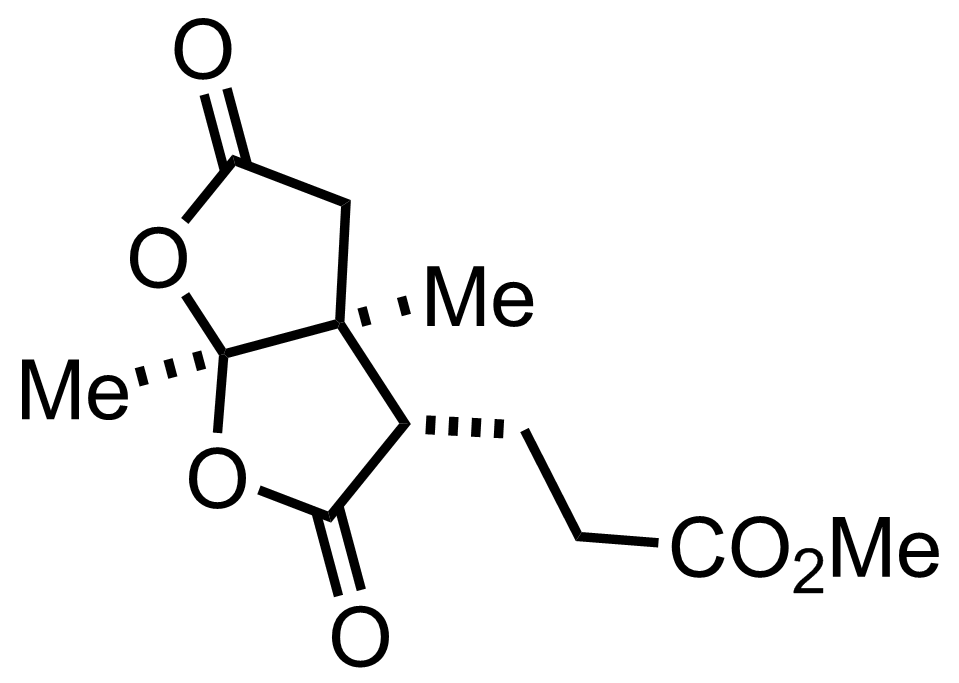
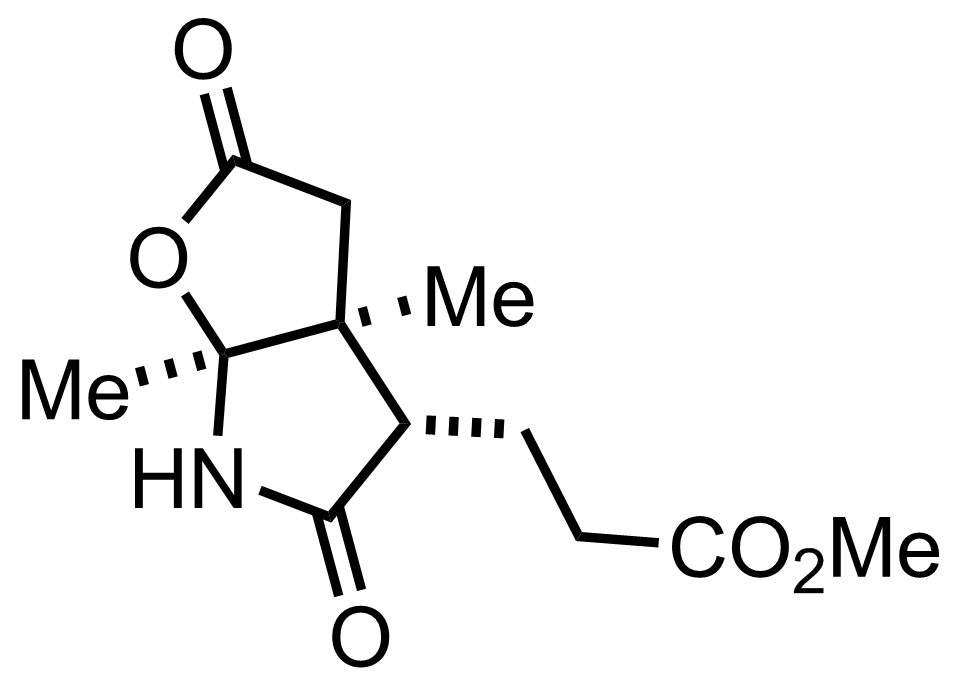
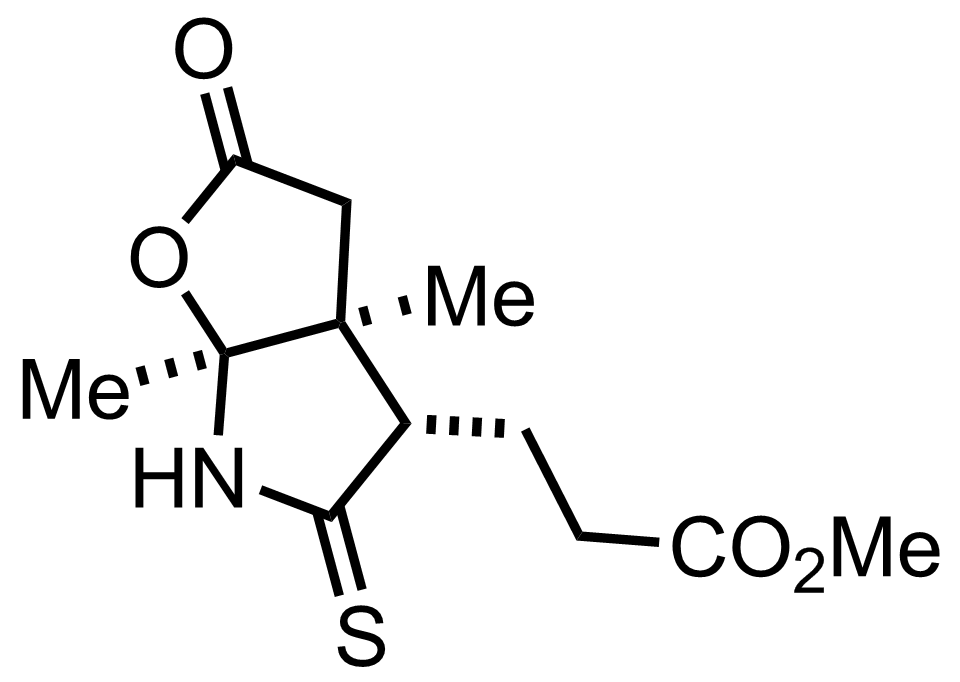
 +
+

 +
+

SnCl4
PhH
RT, 73%
See the Diels-Alder Reaction
"The enantiomers were resolved with (+)- and (-)-phenylethylamine."

CrO3,
H2SO4
Acetone
RT, 75%

SOCl2
77 °C

CH2N2
Dioxane , Et2O
RT


NH3
MeOH
RT, 55%

P2S5
THF
RT, 85%

Part 2 of 7
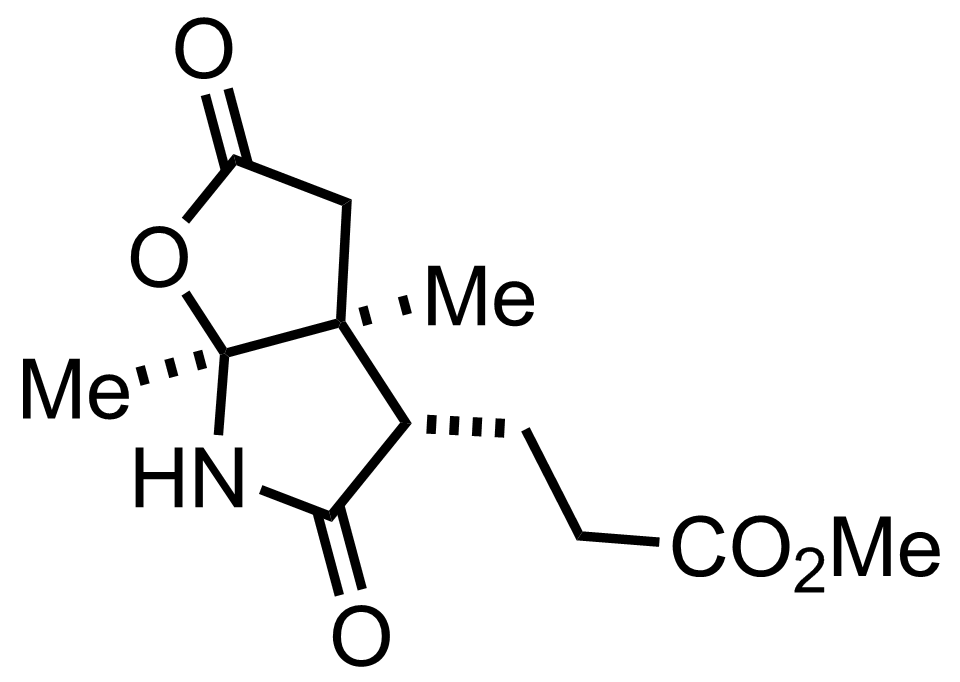
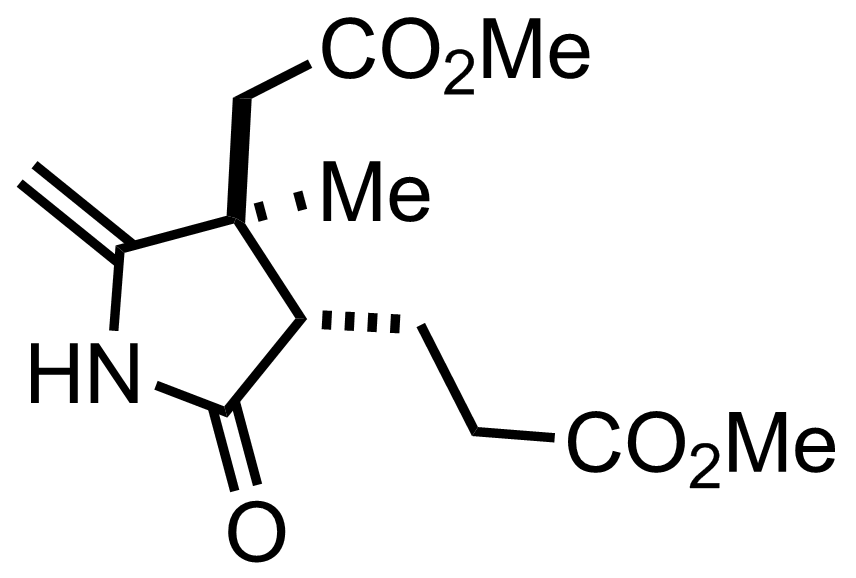
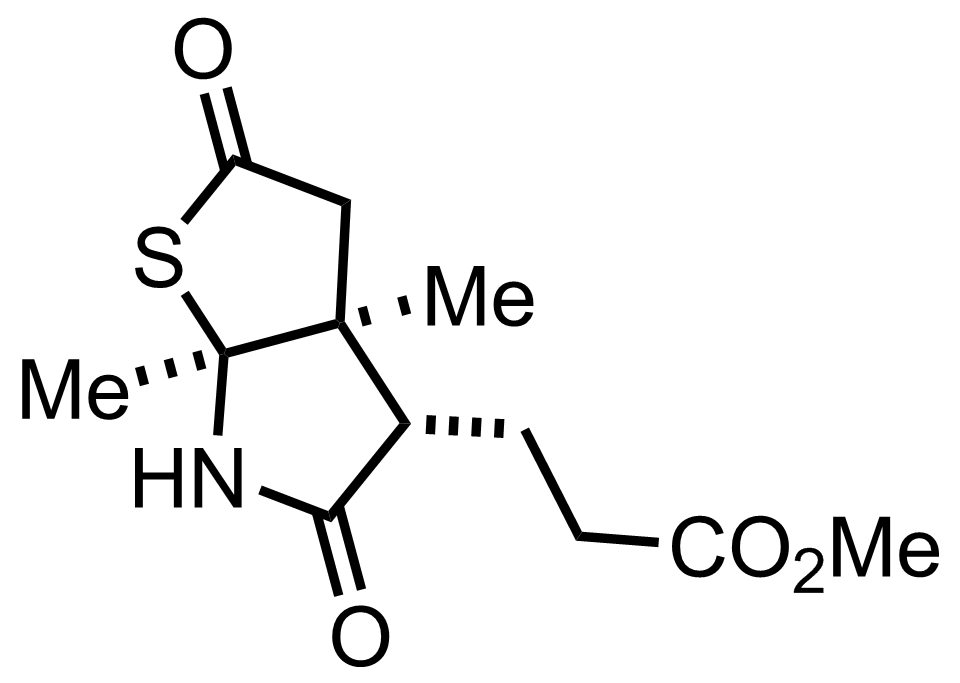
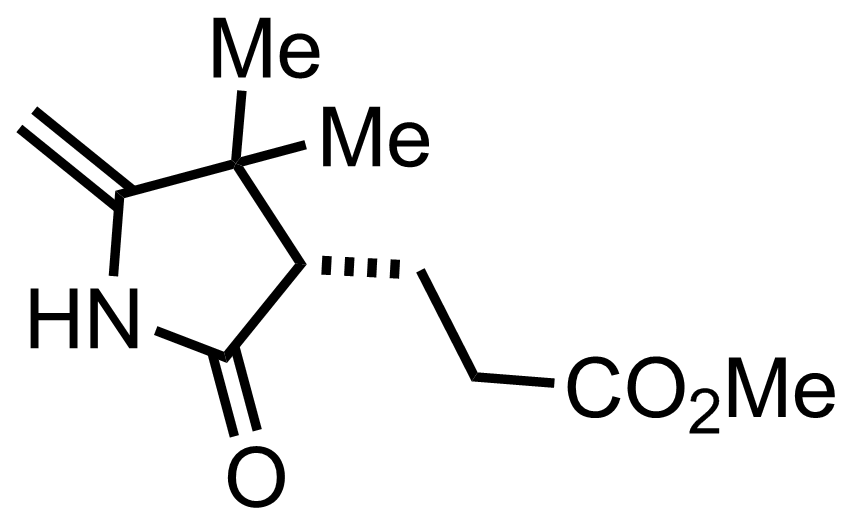

CH2N2,
NaOMe
Et2O, MeOH
92%

H2S
CF3CO2H
RT, 78%

(Ph3P)3RhCl
PhMe
110 °C, 30%

Part 3 of 7
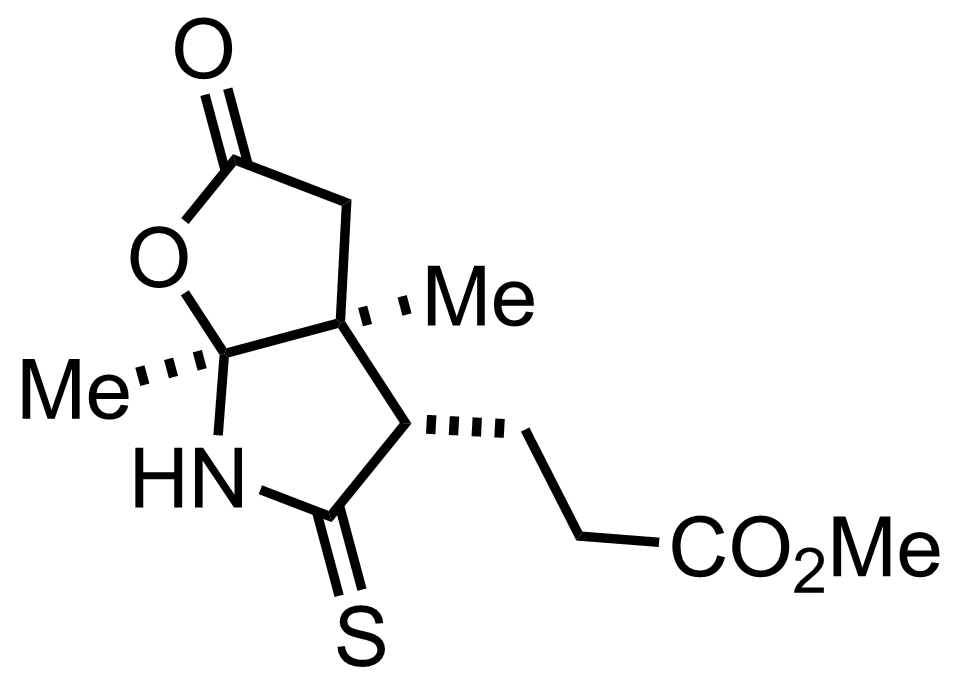 +
+
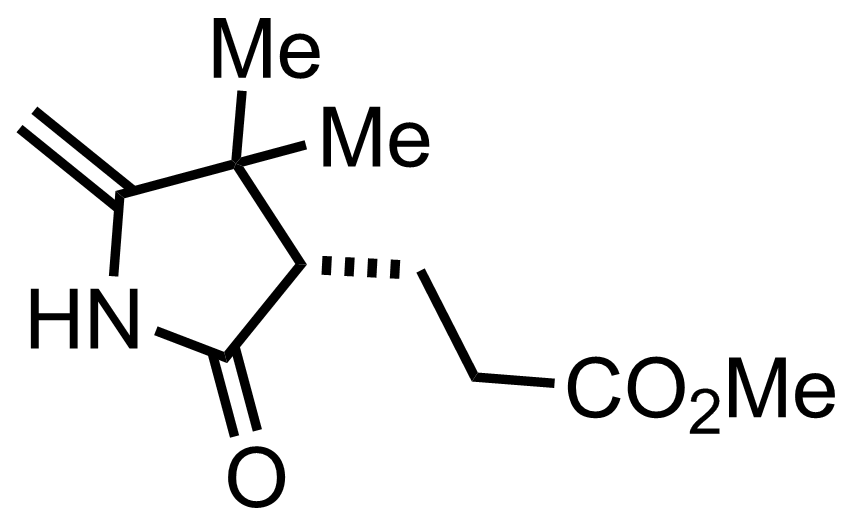
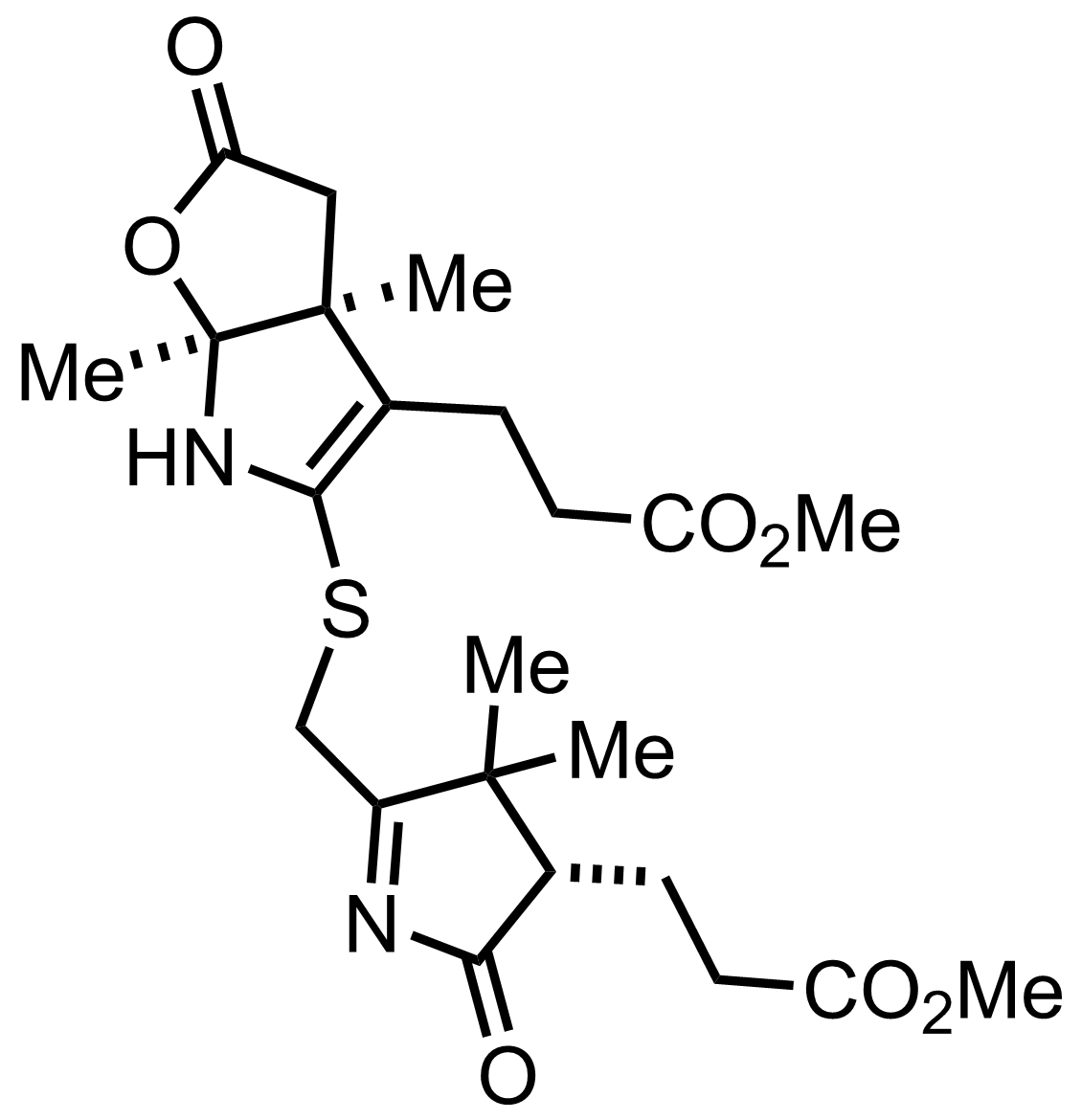
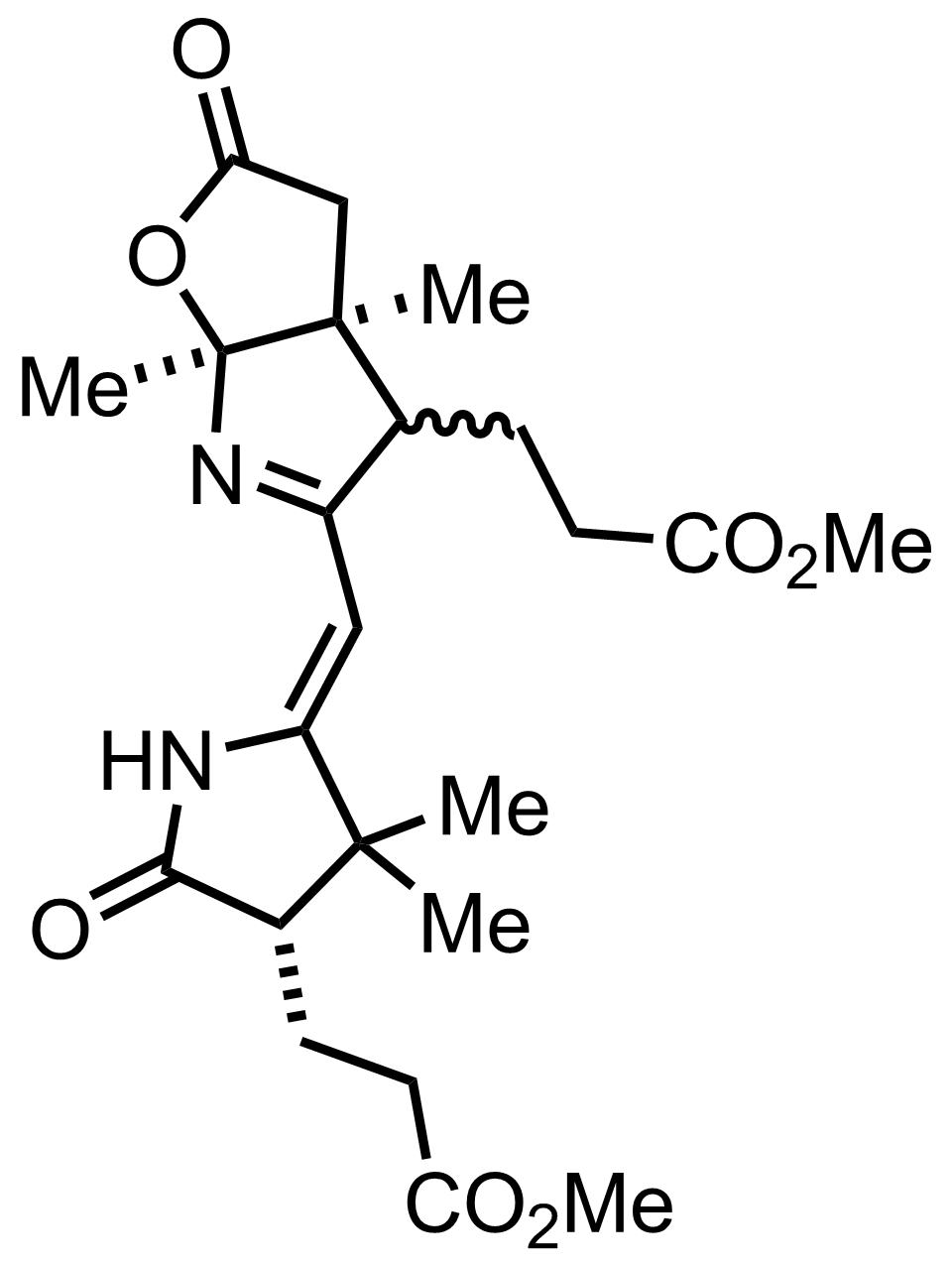
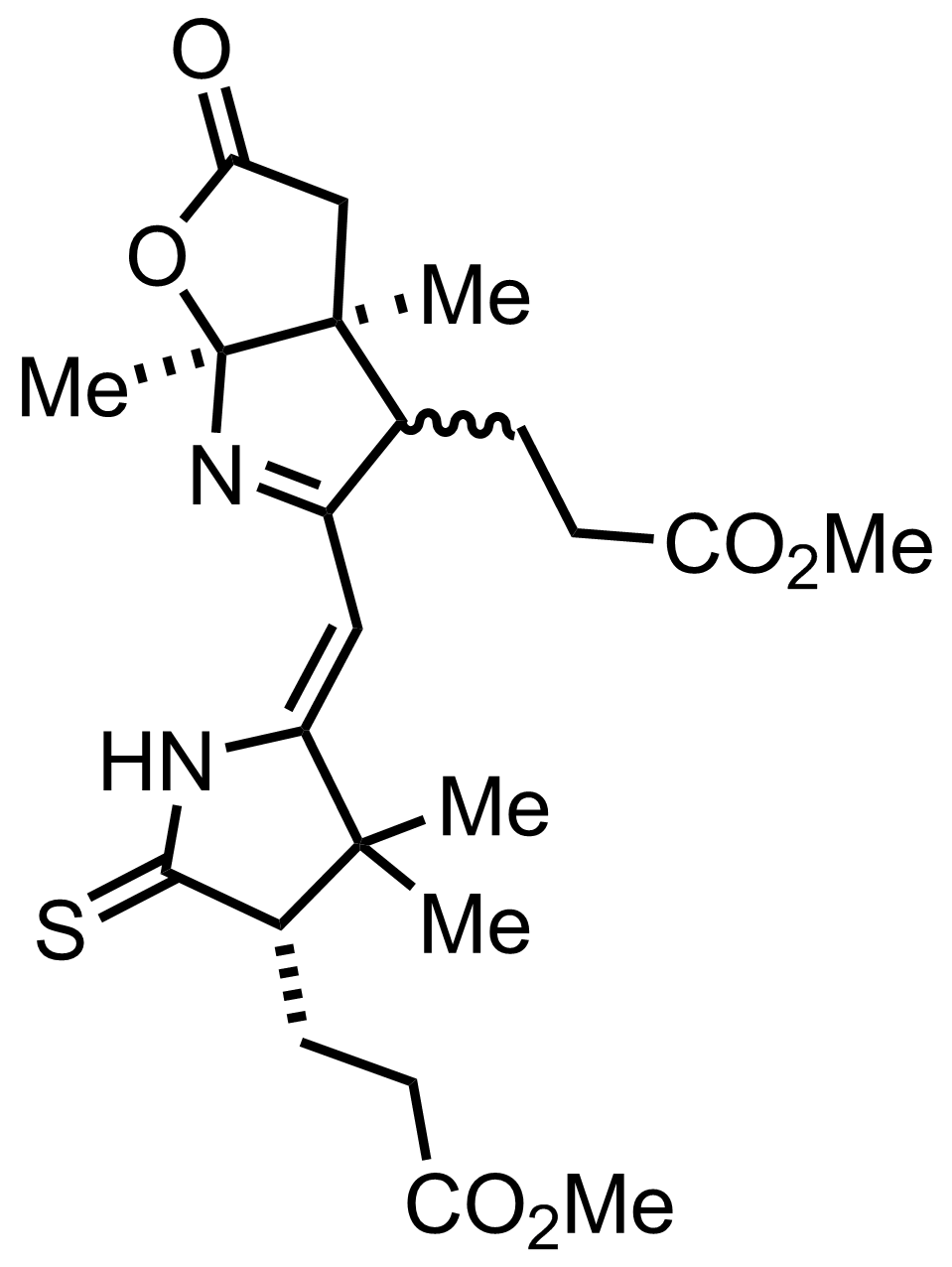
 +
+

HCl,
Bz2O
CH2Cl2
RT


P2S5,
4-Methylpyridine
Xylene(s)
130 °C, 84%

Part 4 of 7
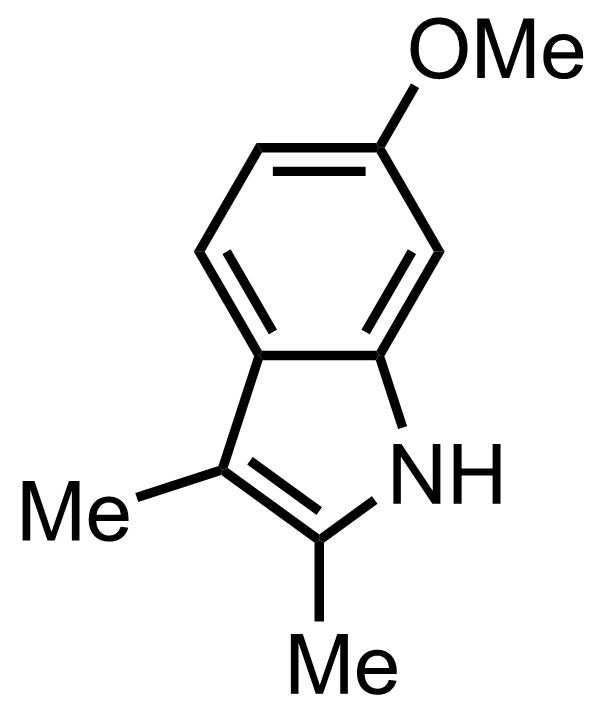
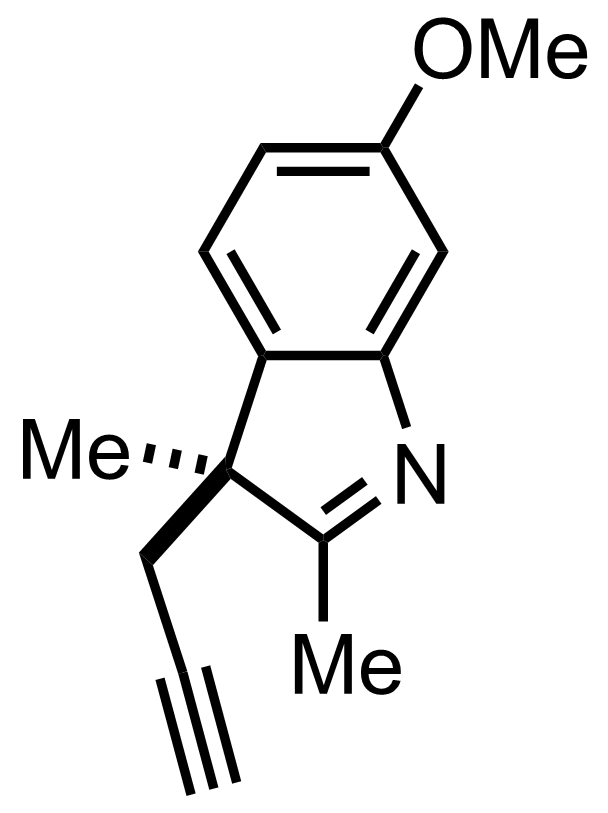
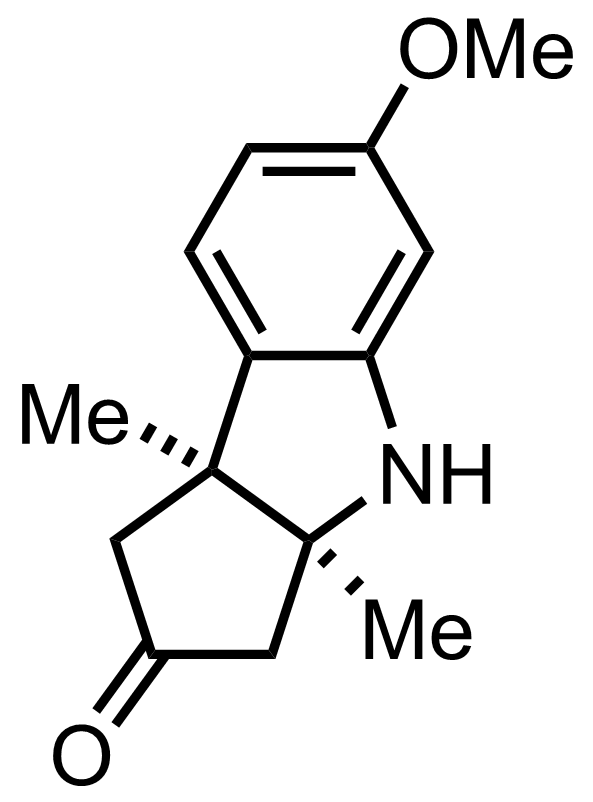 +
+
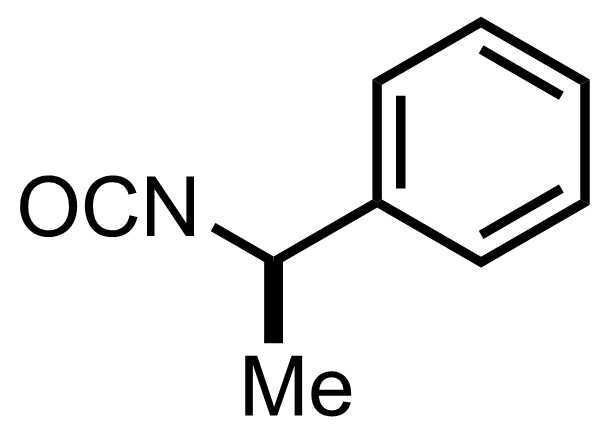
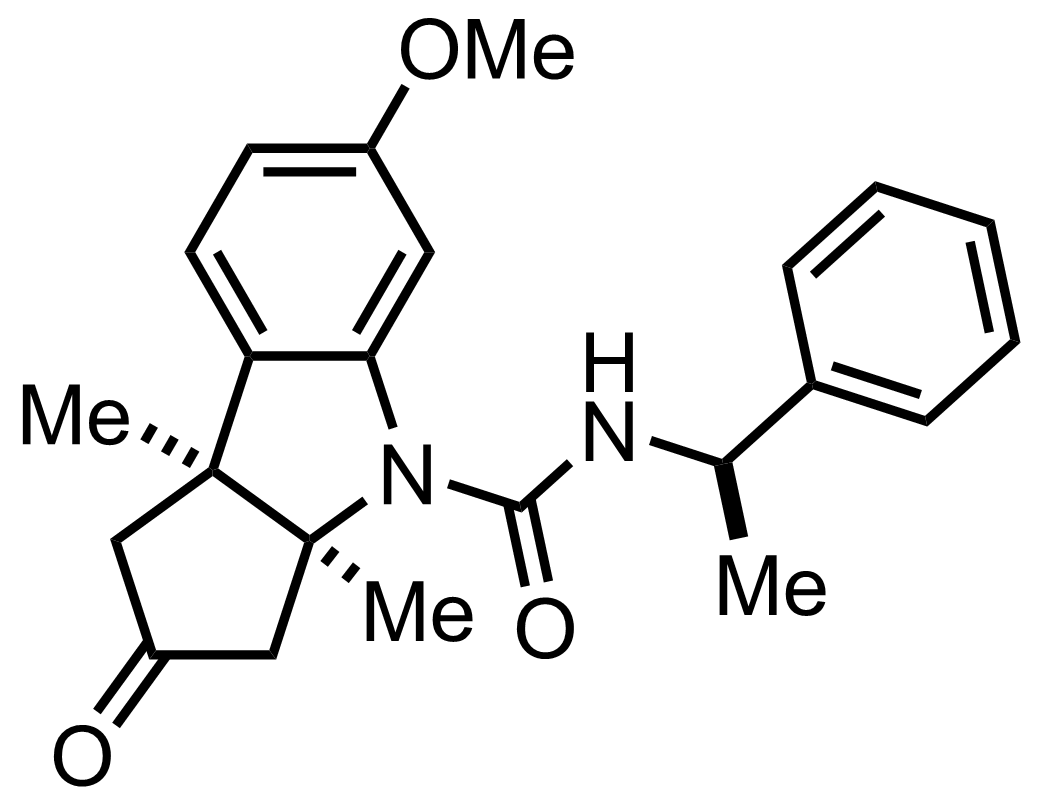
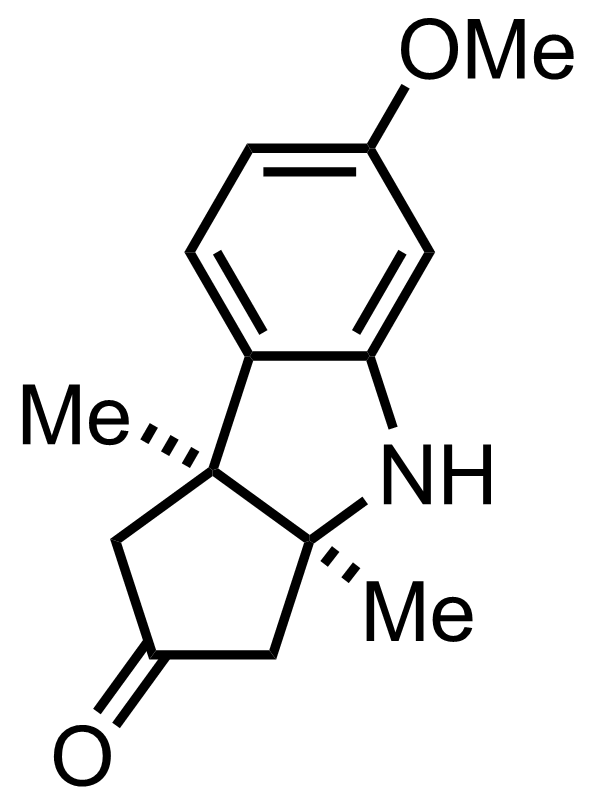

MeMgI,
Propargyl bromide

 +
+

""the diastereomeric ureas resulting from the combination of the racemic tricyclic ketone with optically active alpha-phenylethylisocyanate are very readily separable""

""and are readily re-converted to the parent active tricyclic ketones when pyrolyzed""

Part 5 of 7
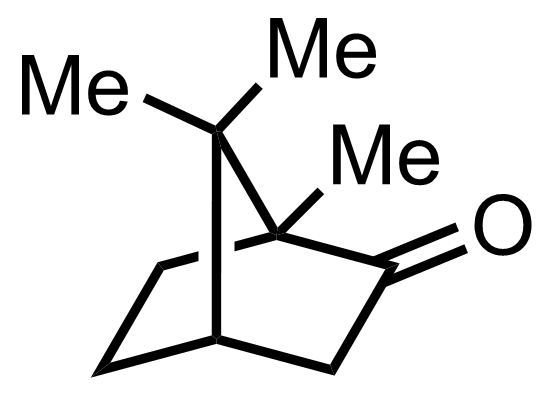
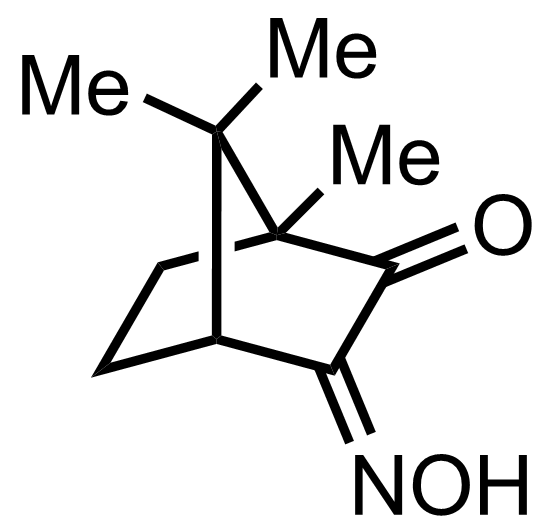
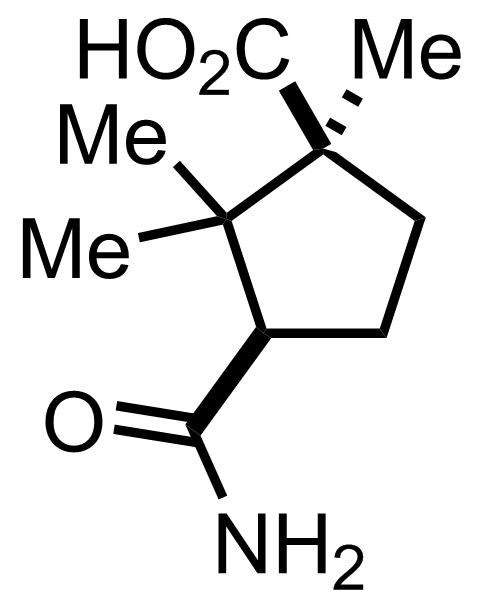
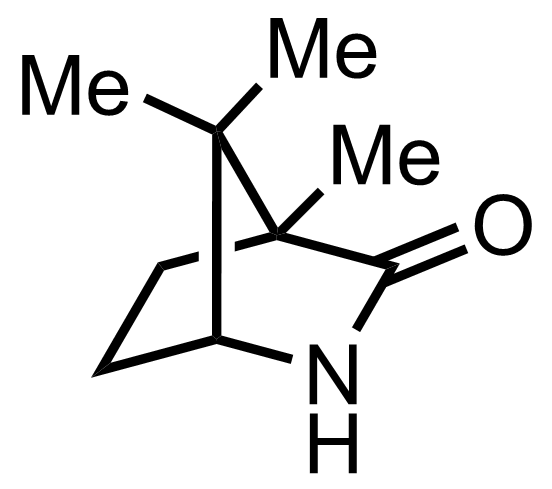
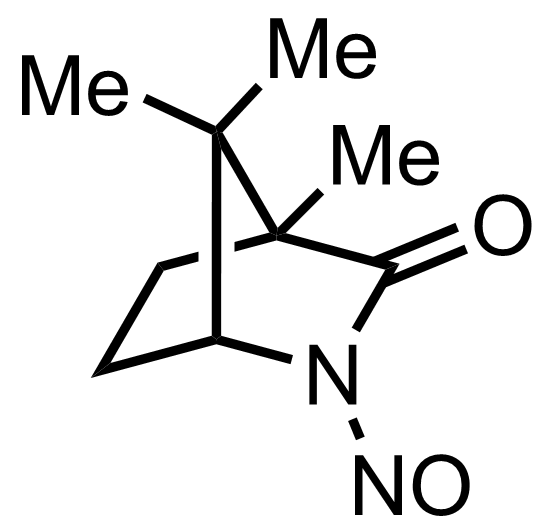
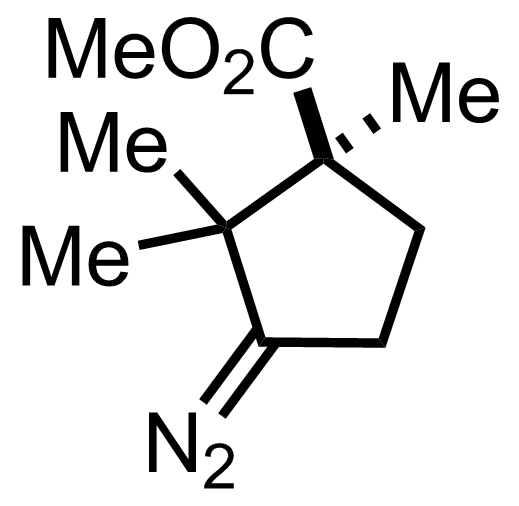
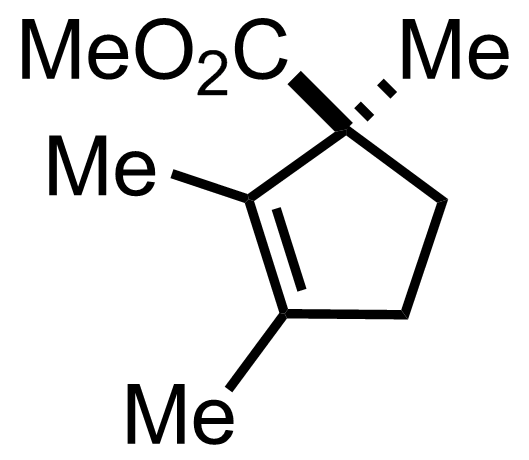
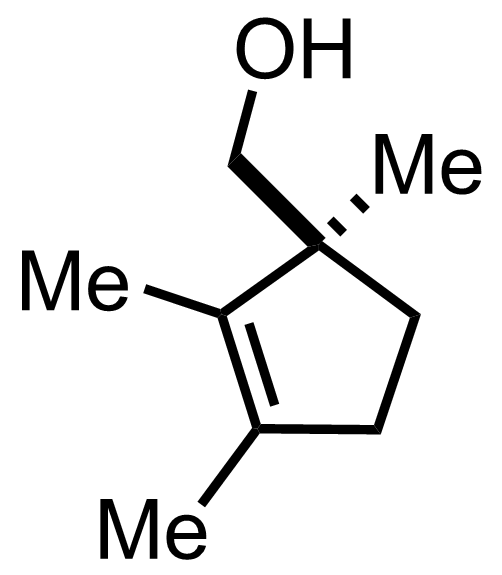
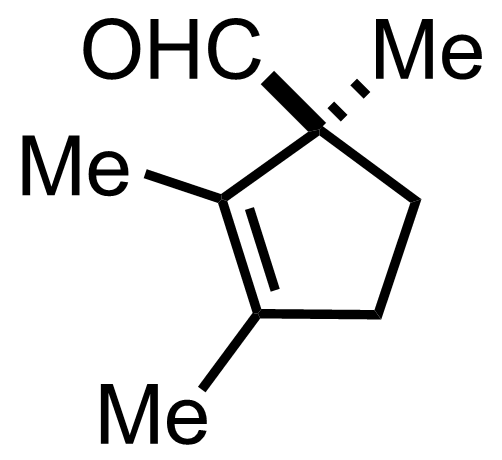 +
+
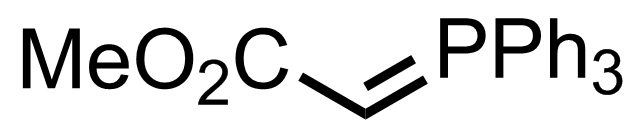
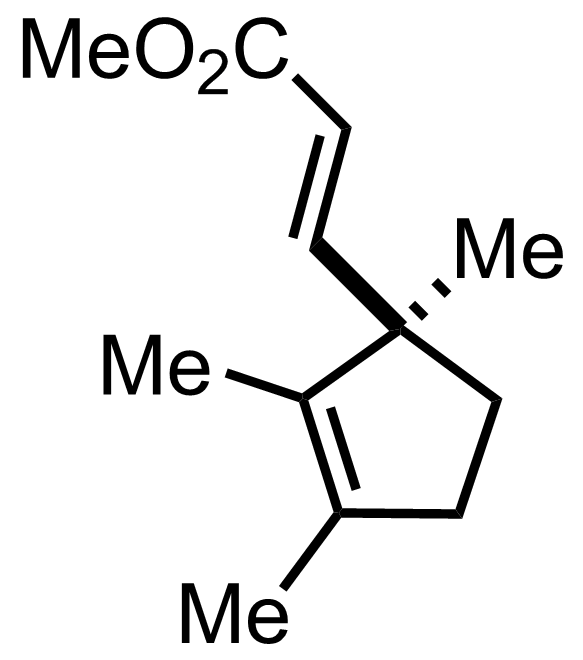
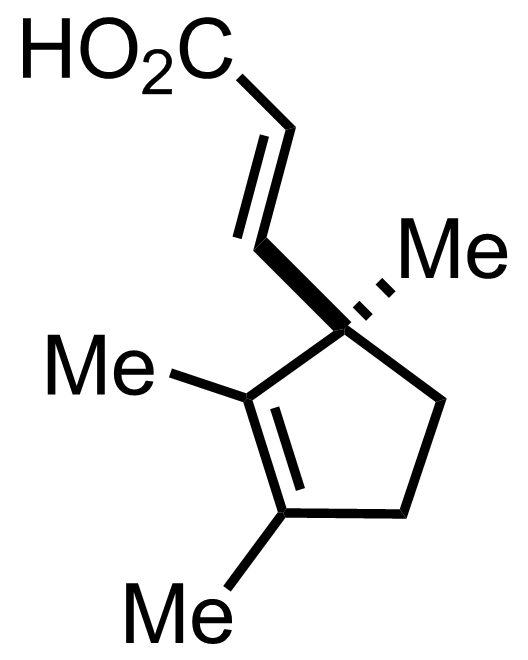
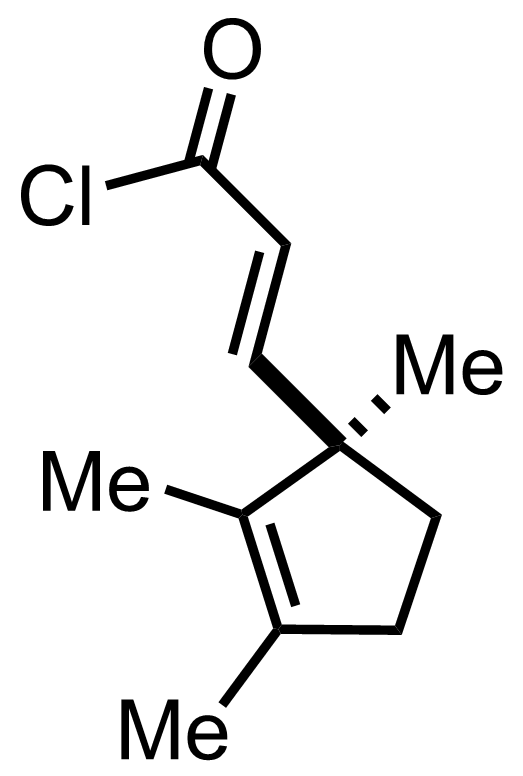

"No experimental details were reported for this whole part."






LiAlH4

CrO3
 +
+




Part 6 of 7
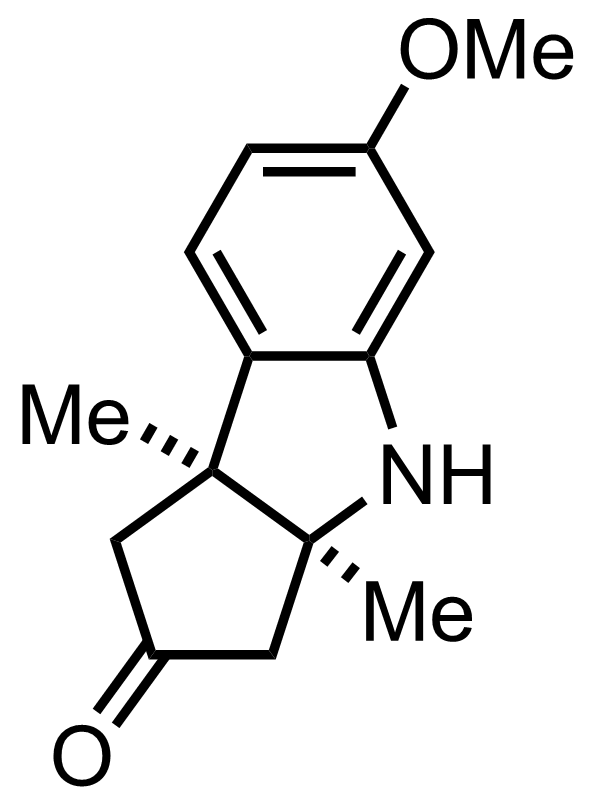 +
+
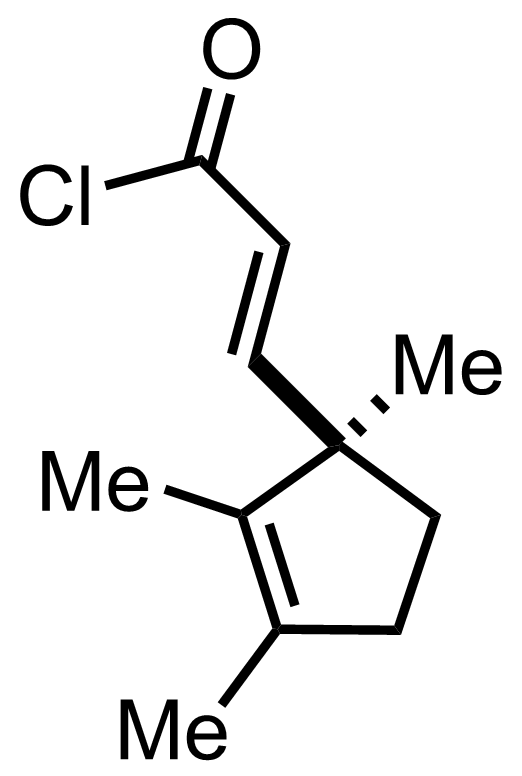
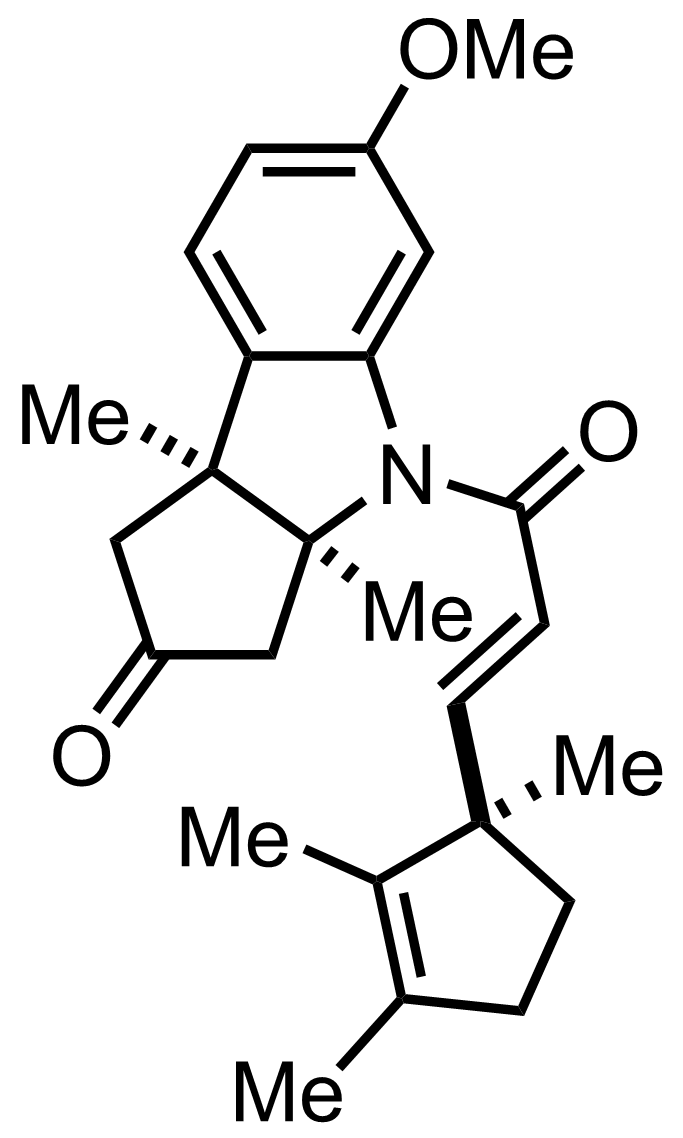
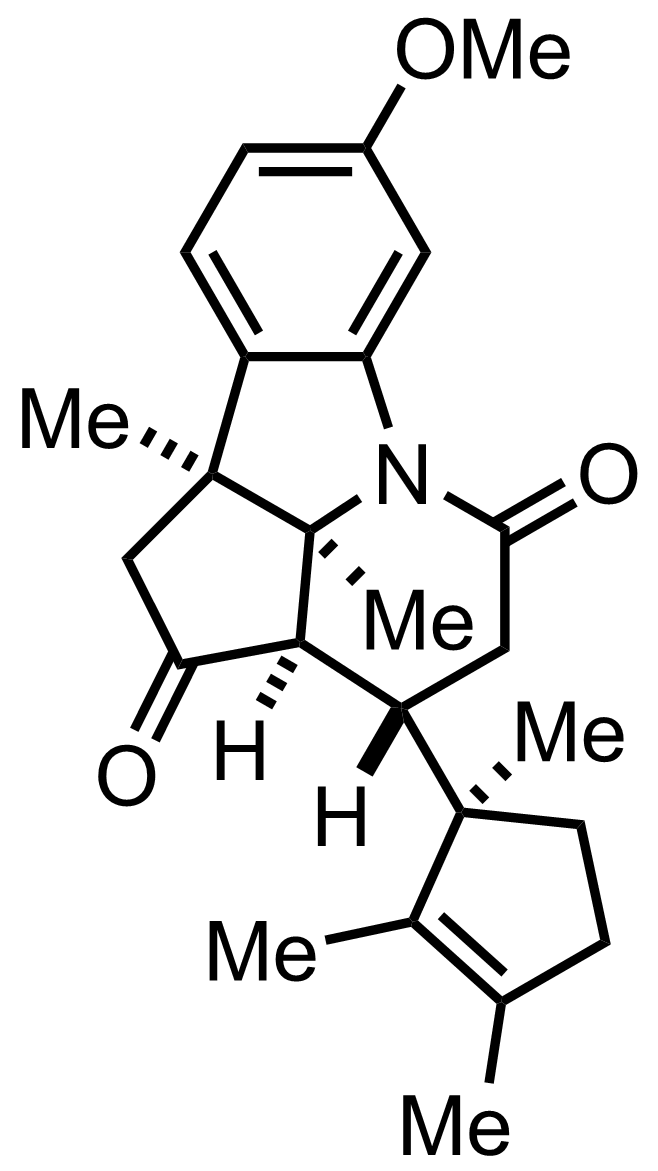
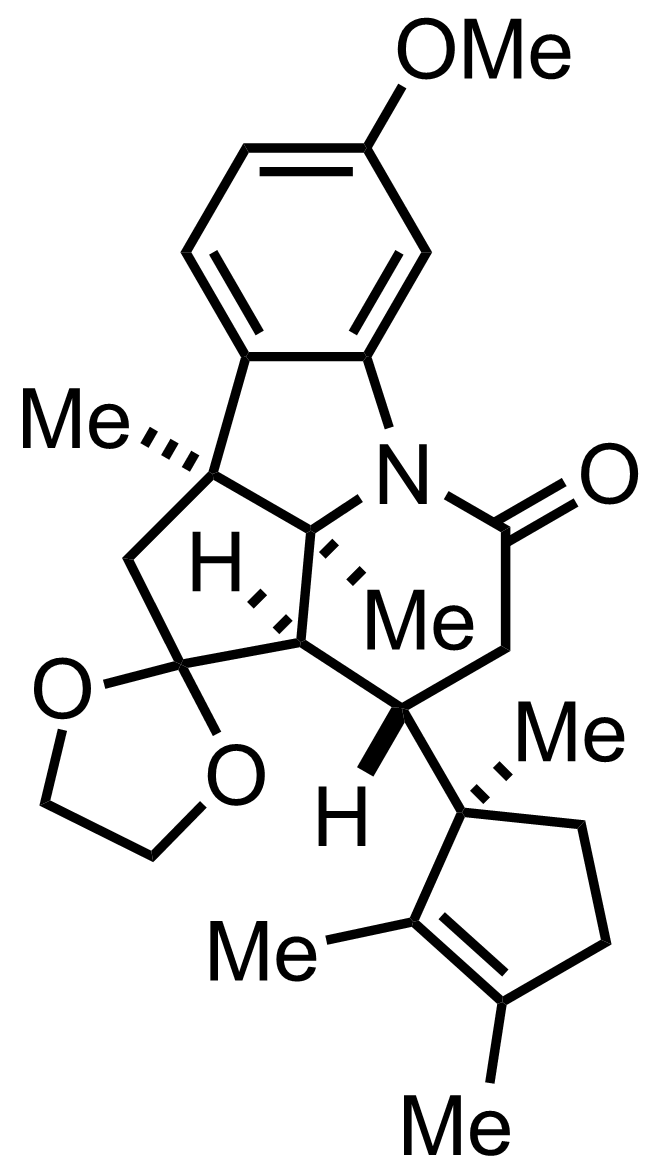
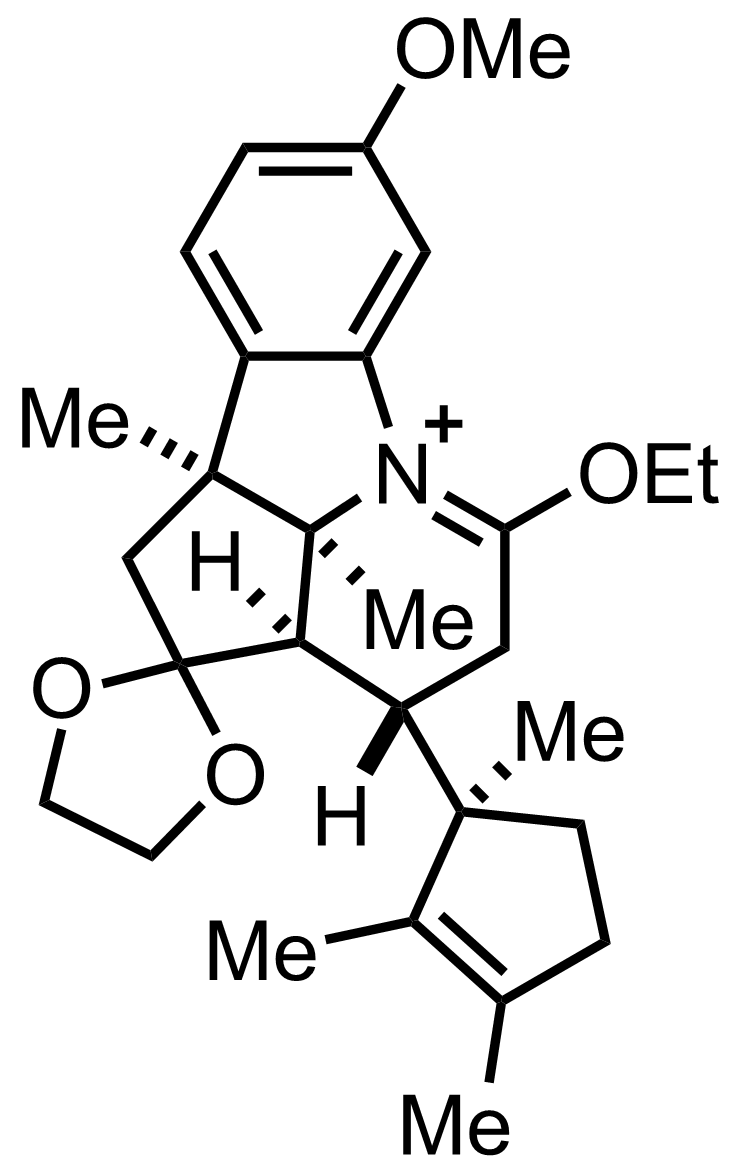
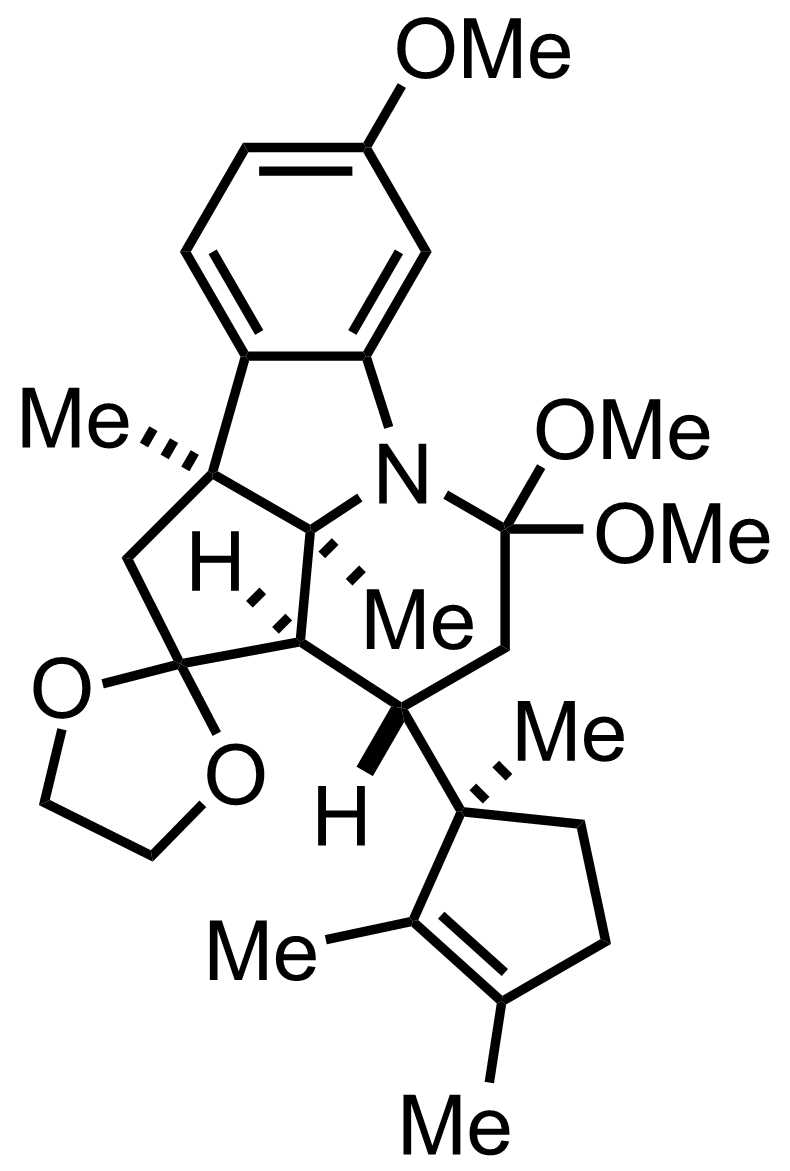
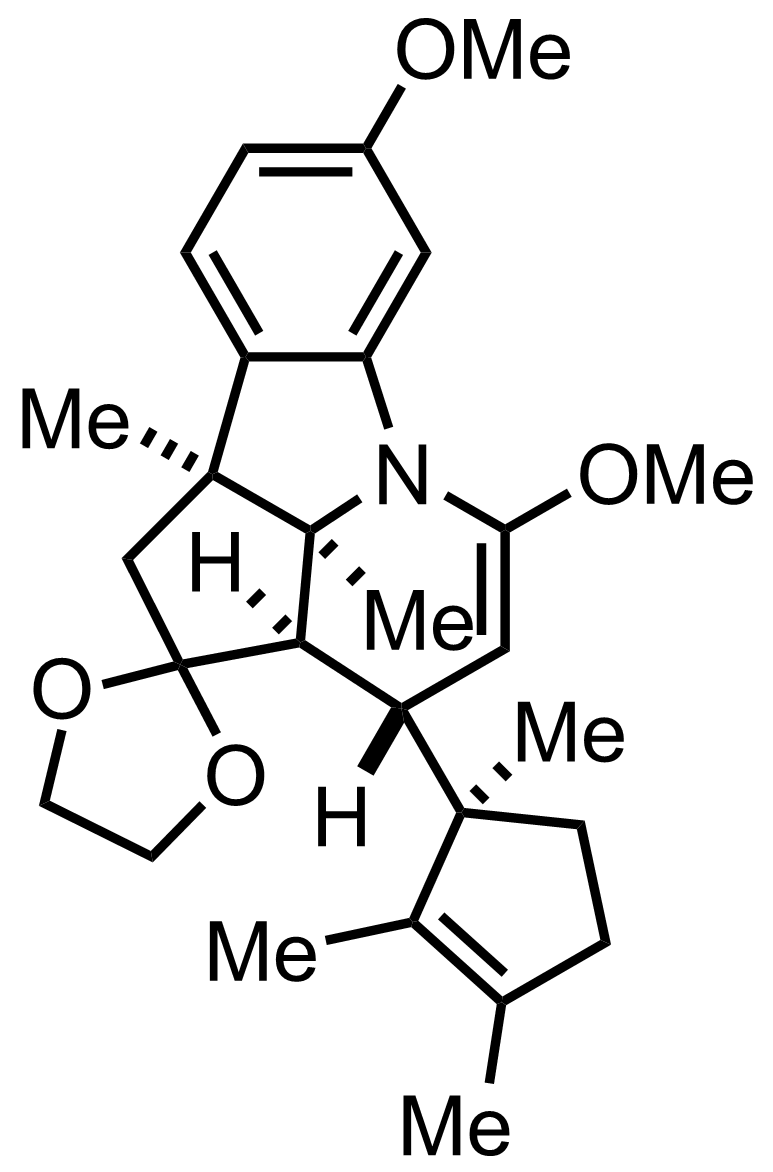
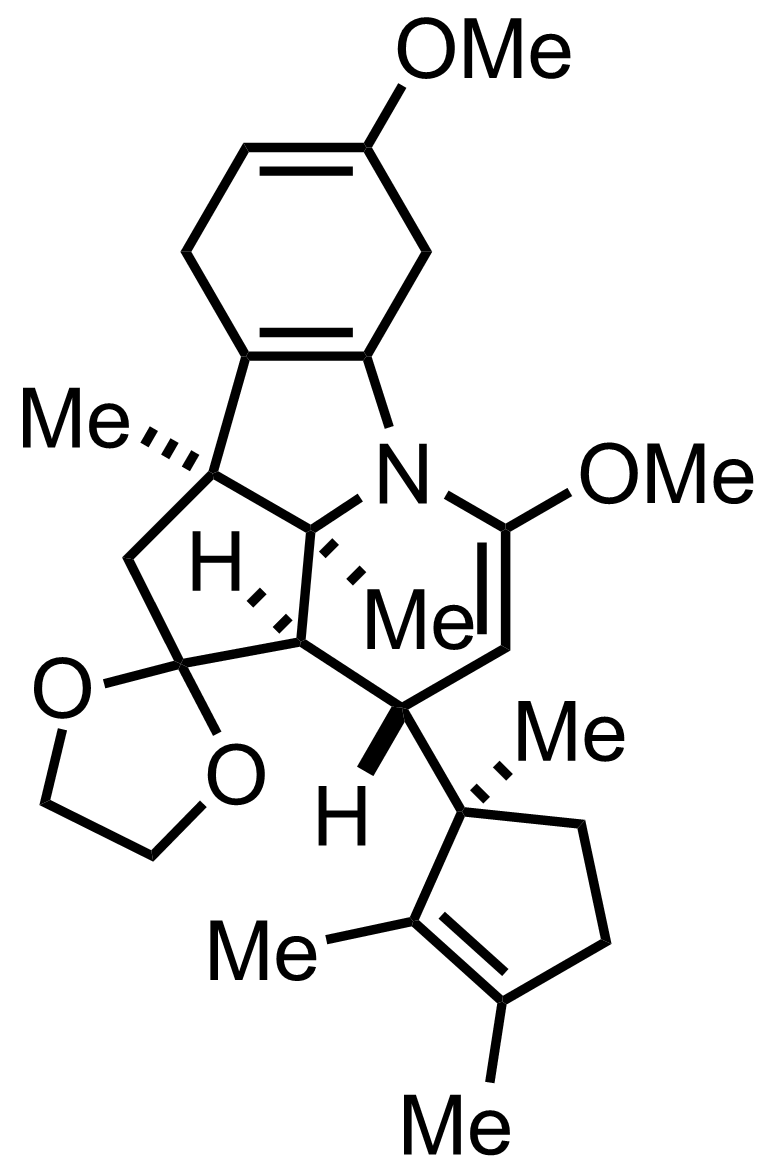
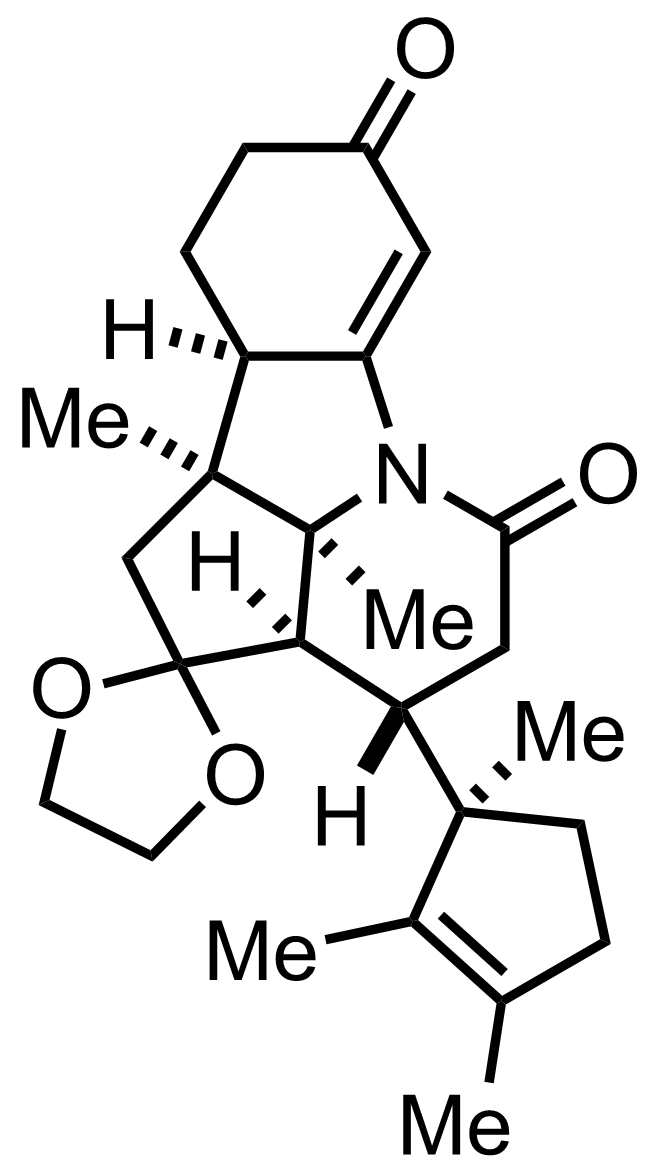
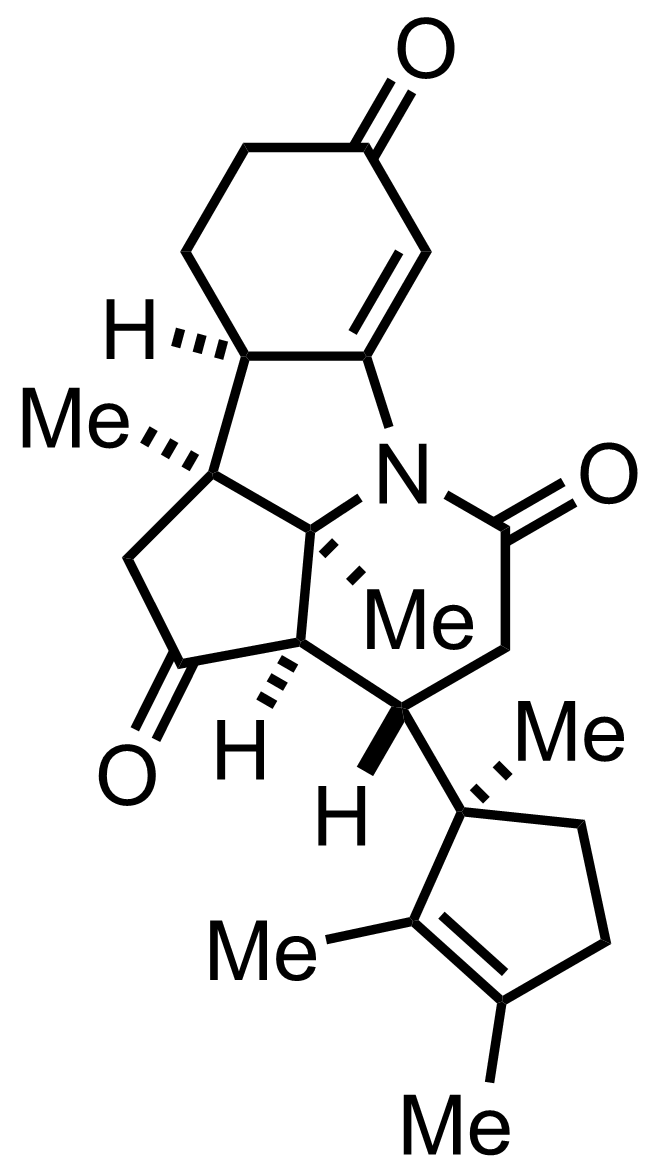
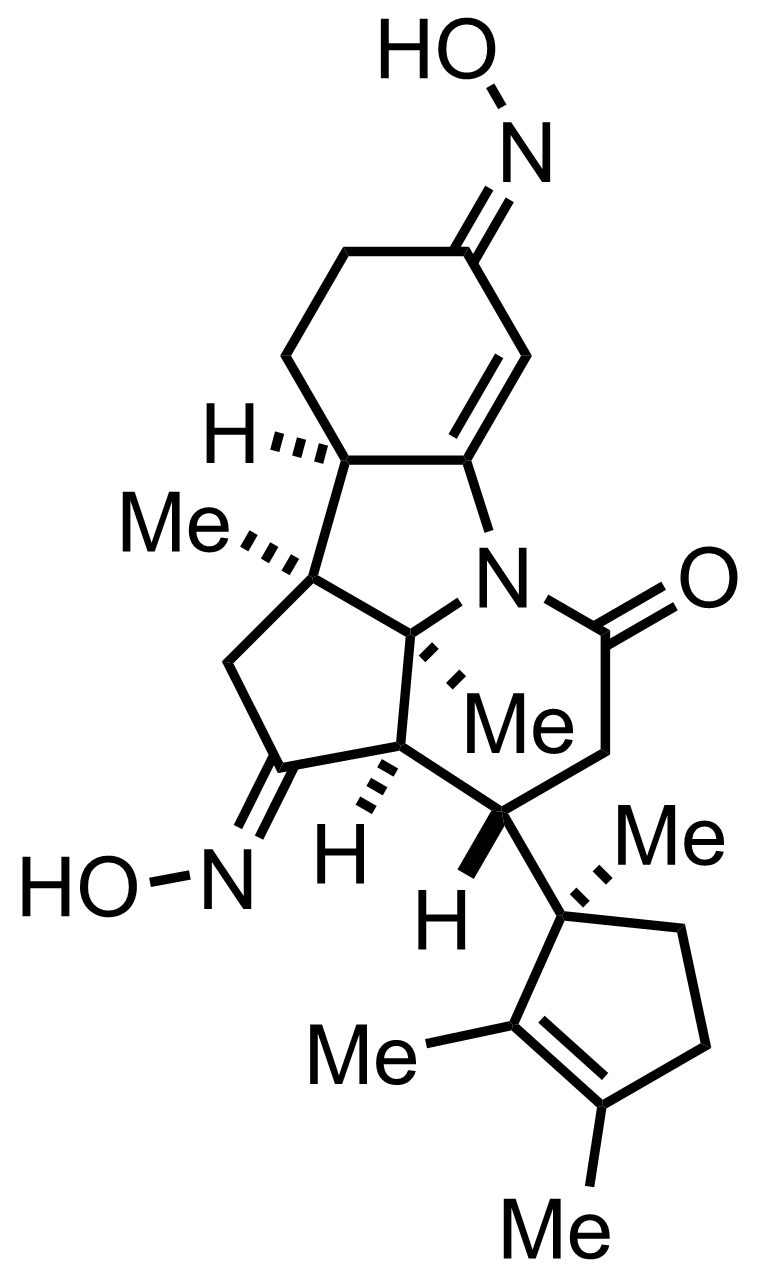
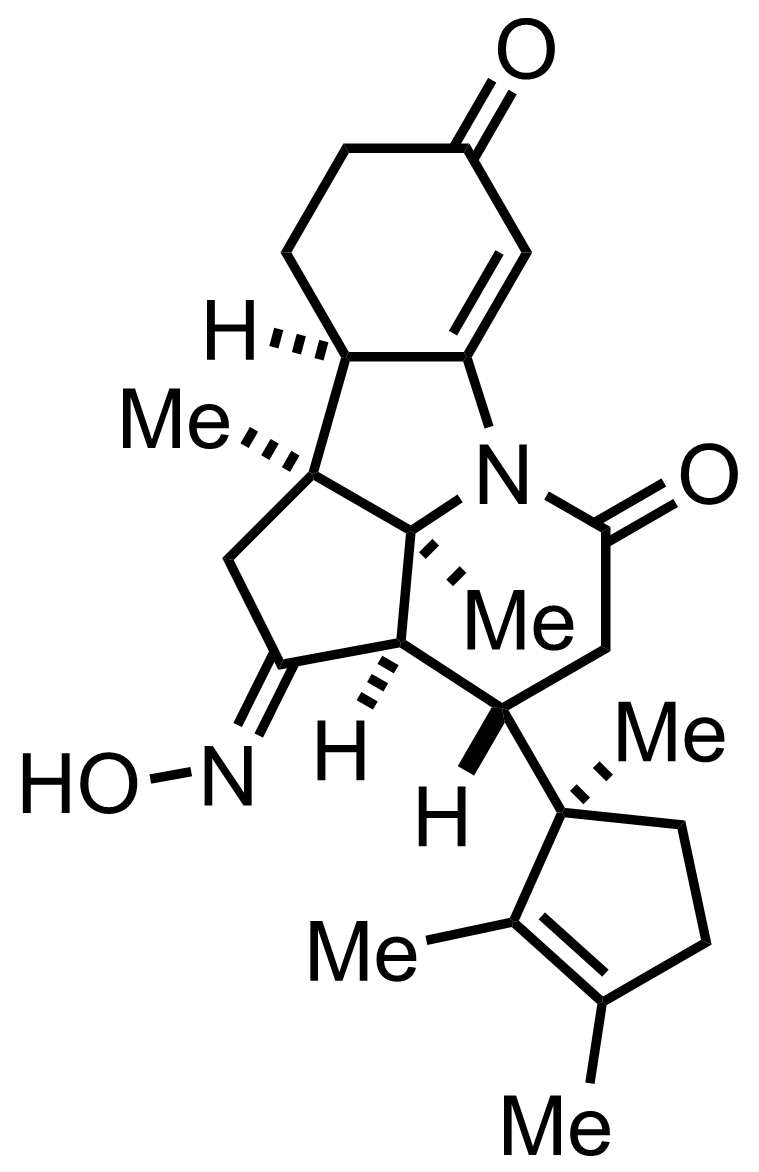
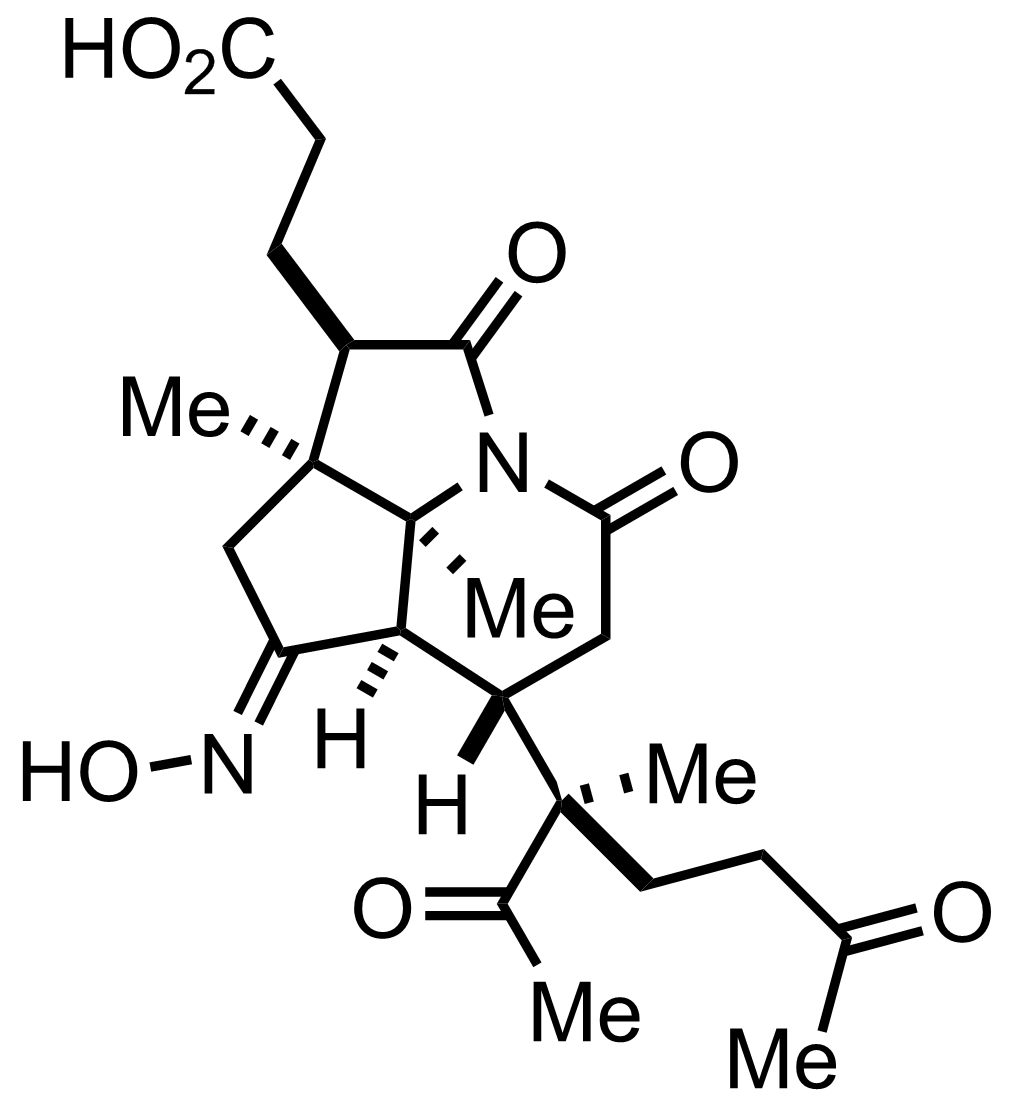
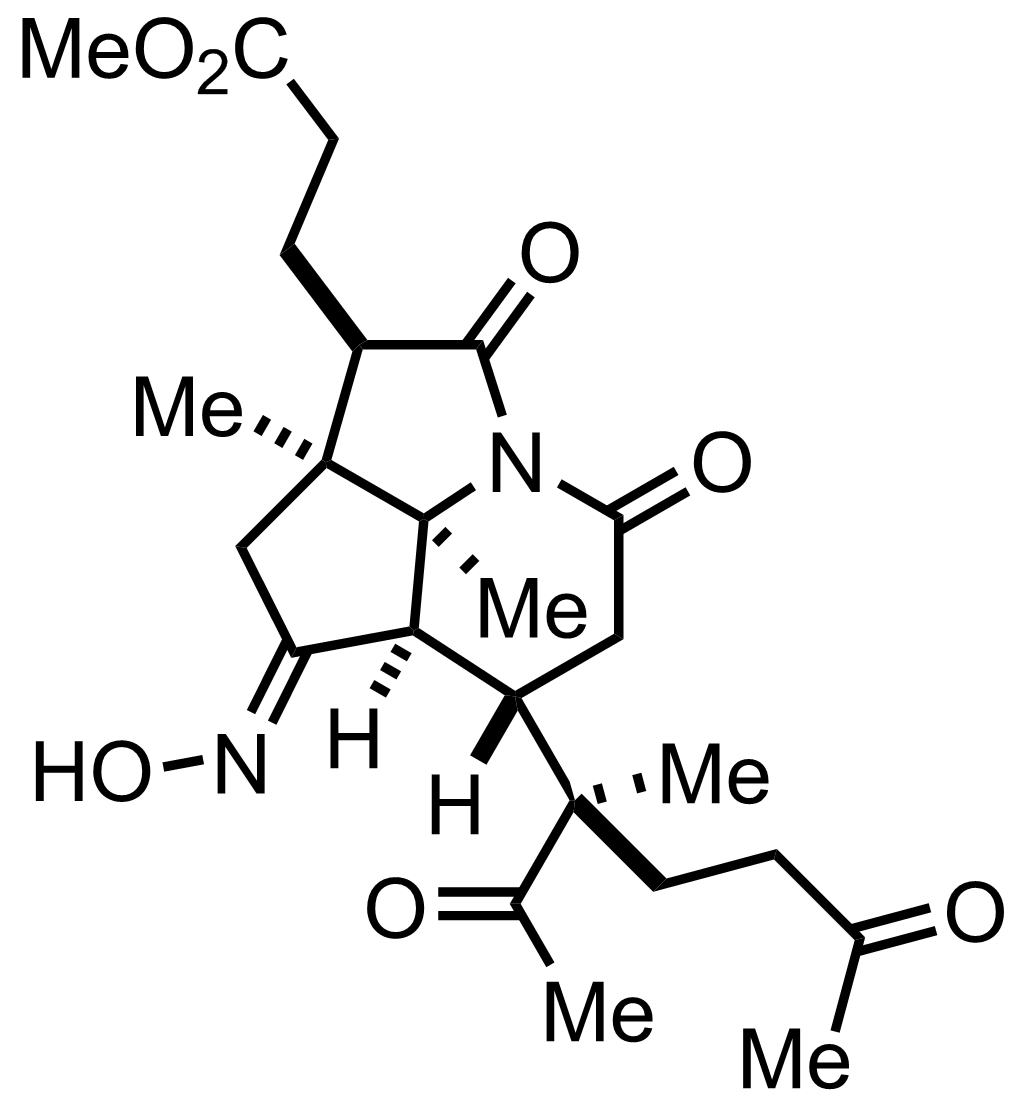
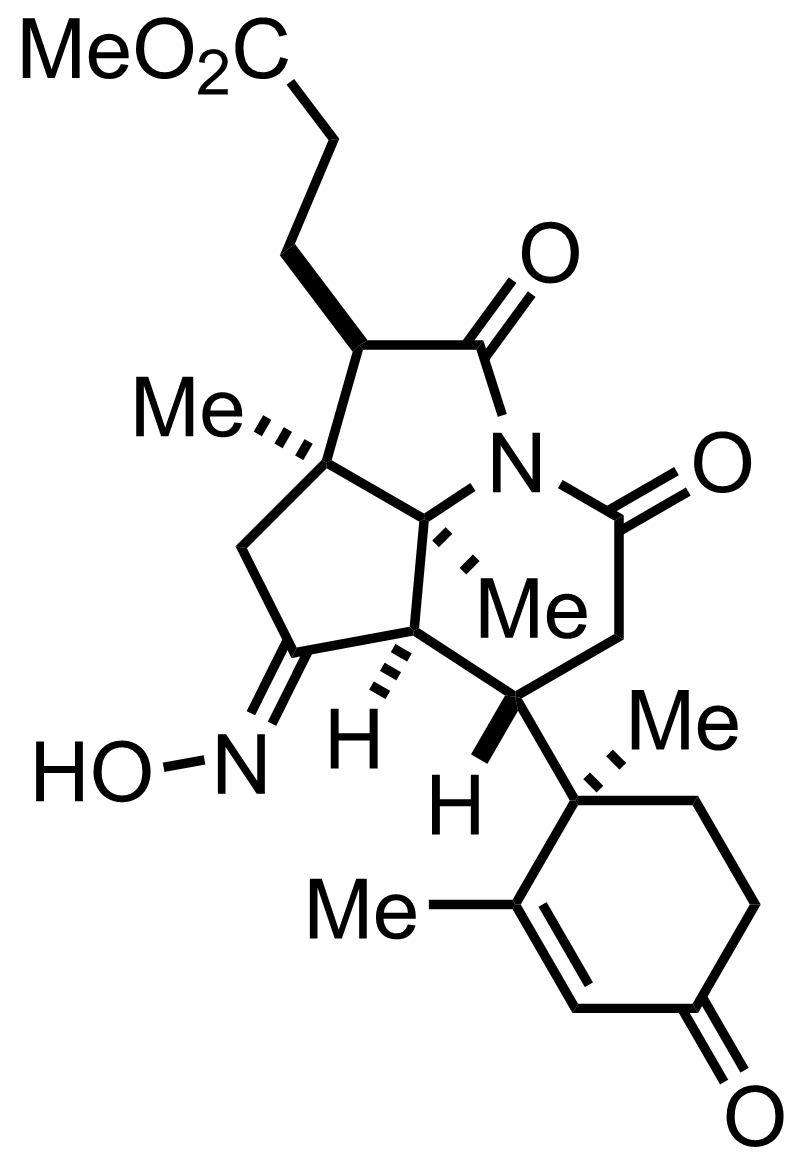
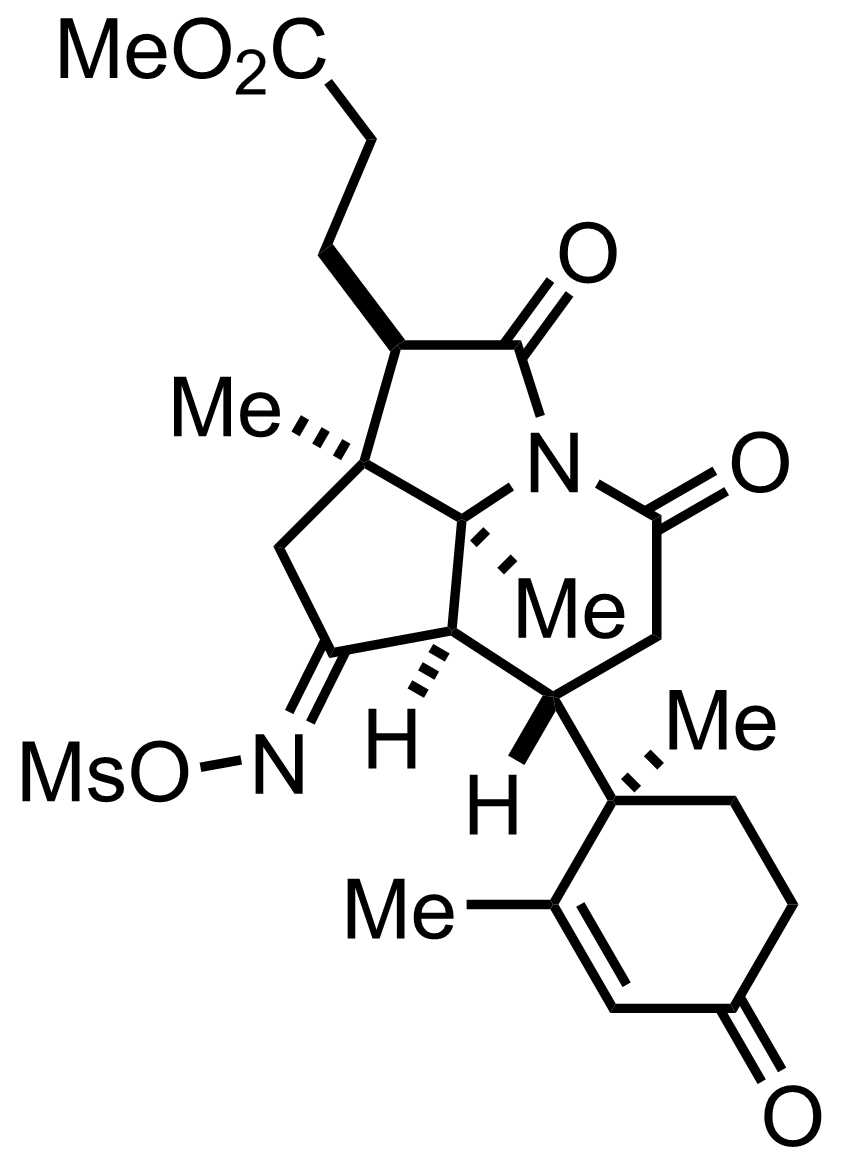
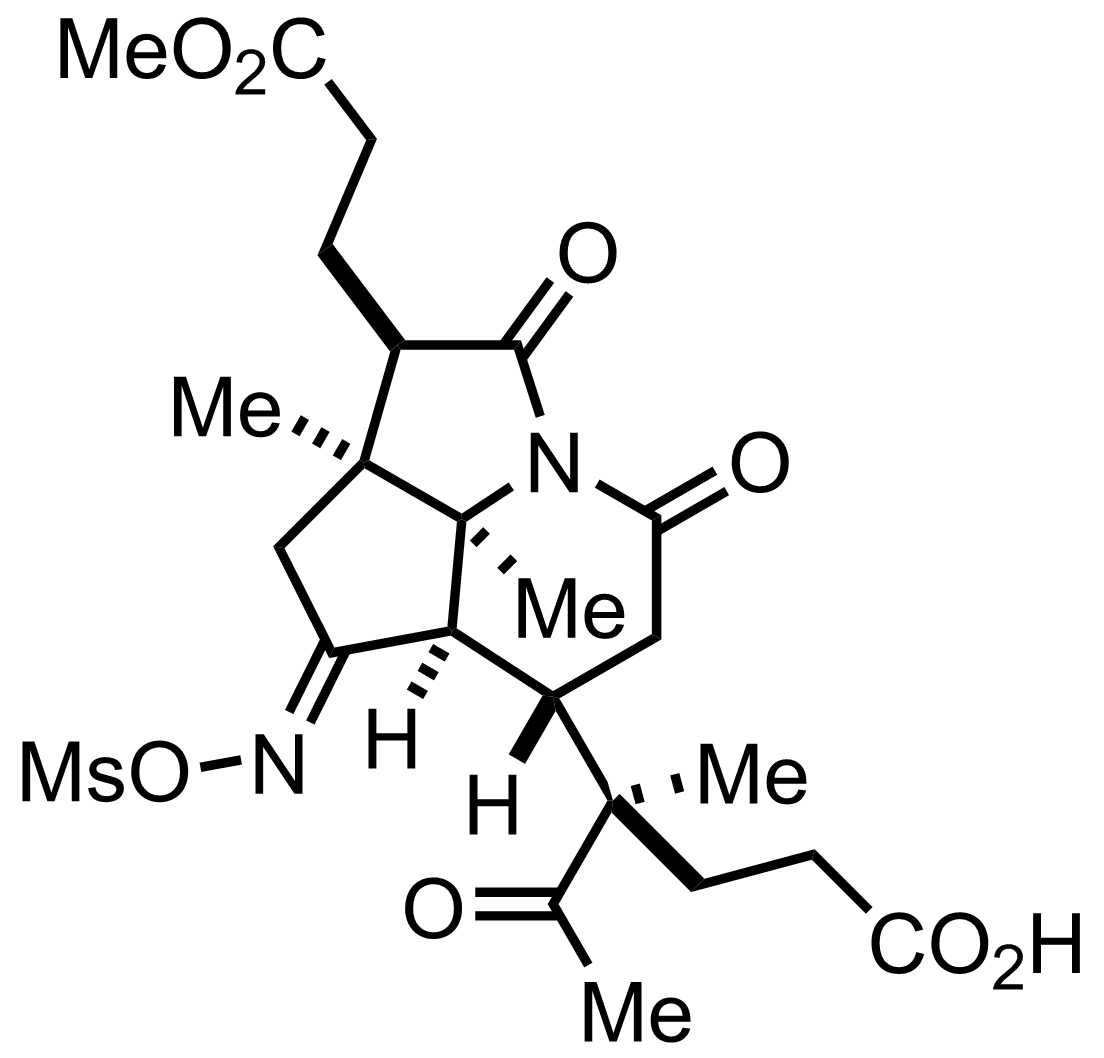
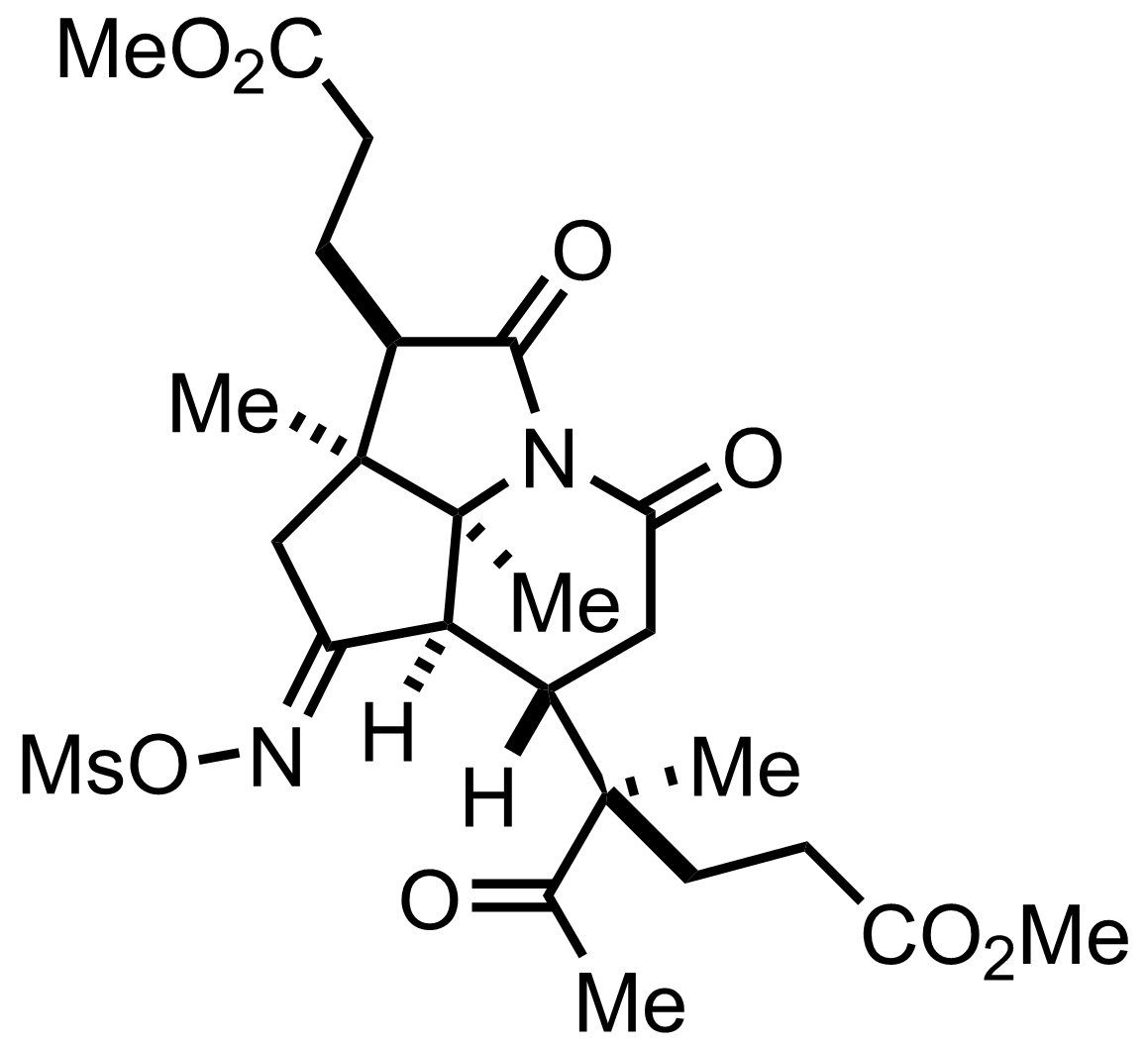
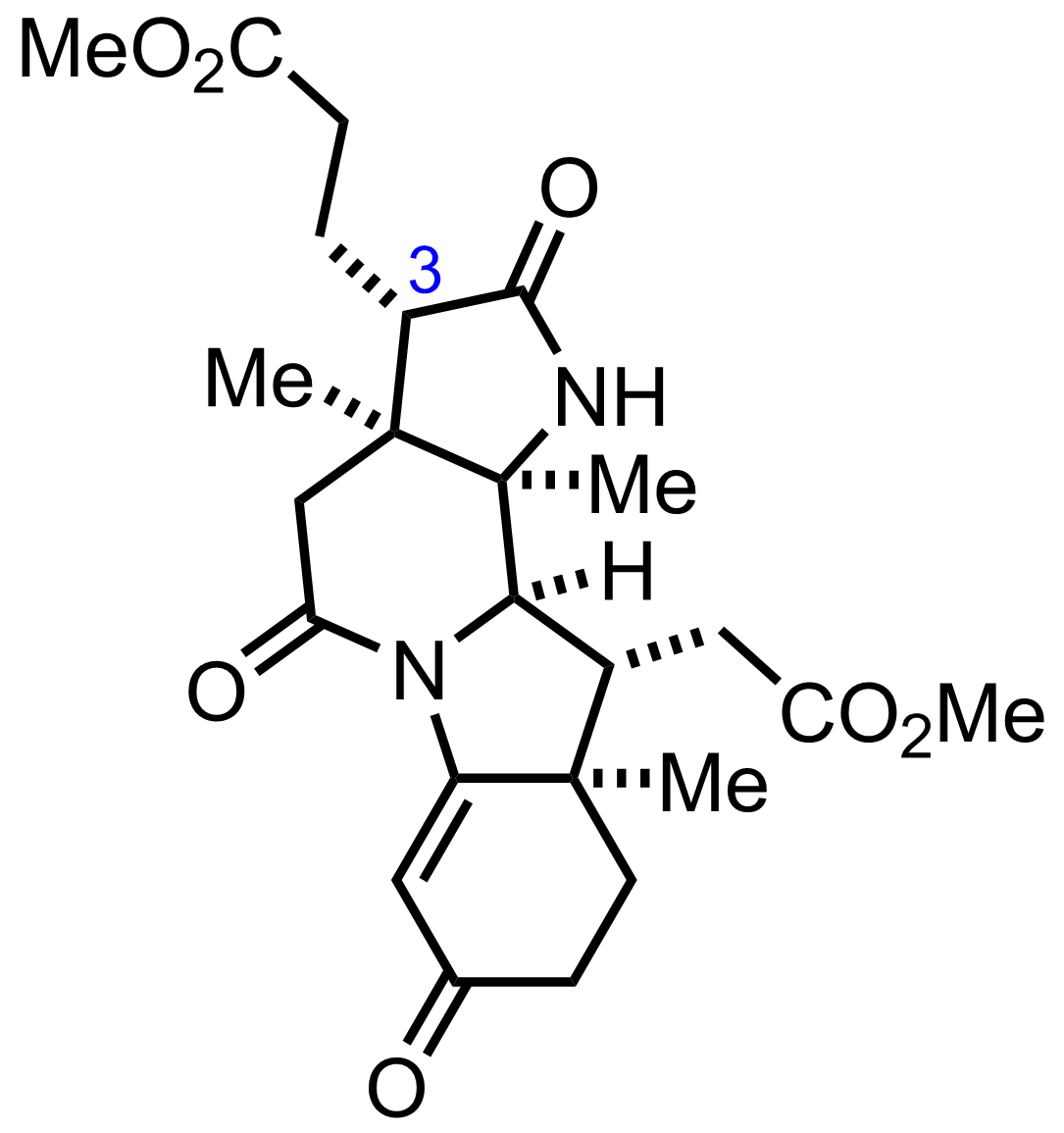
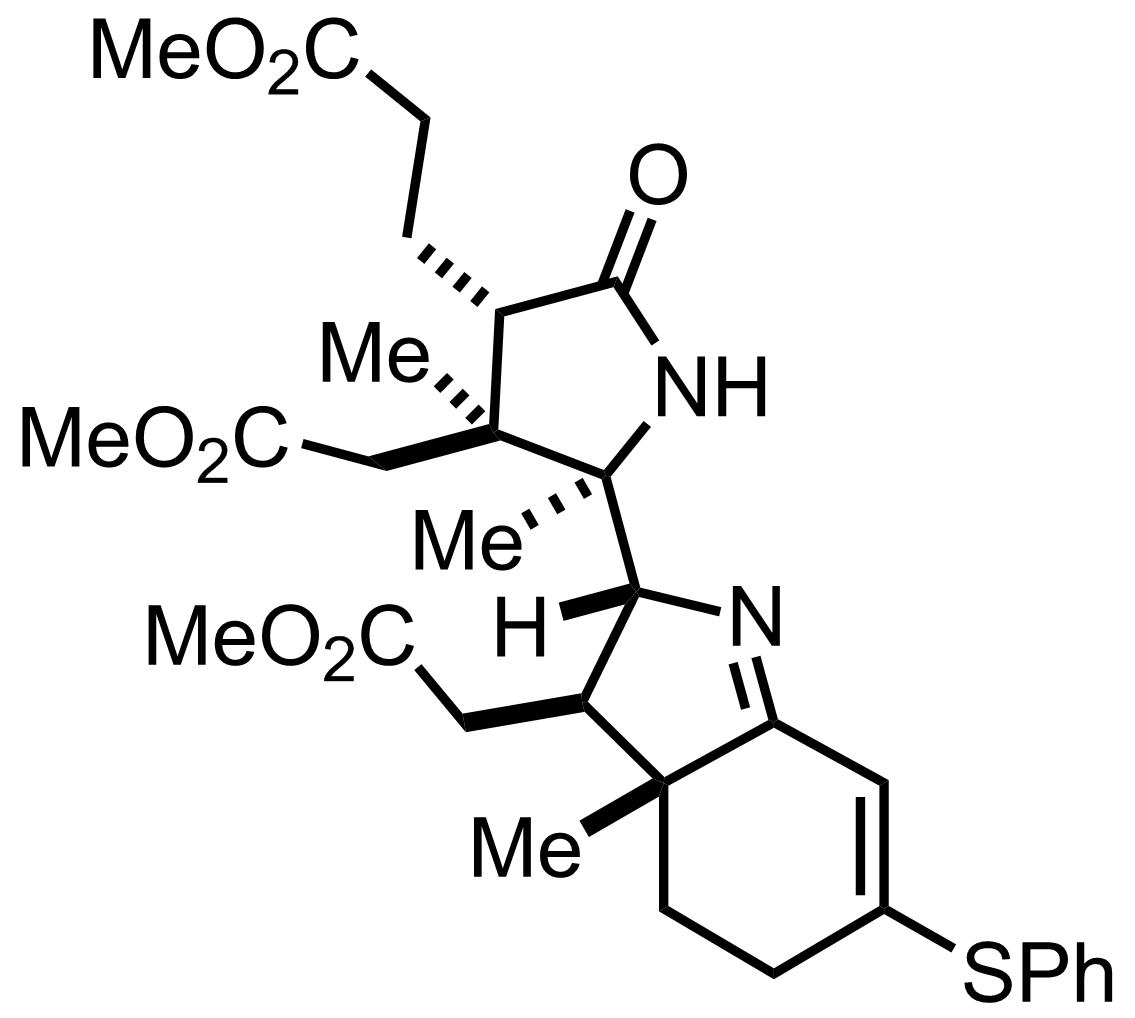
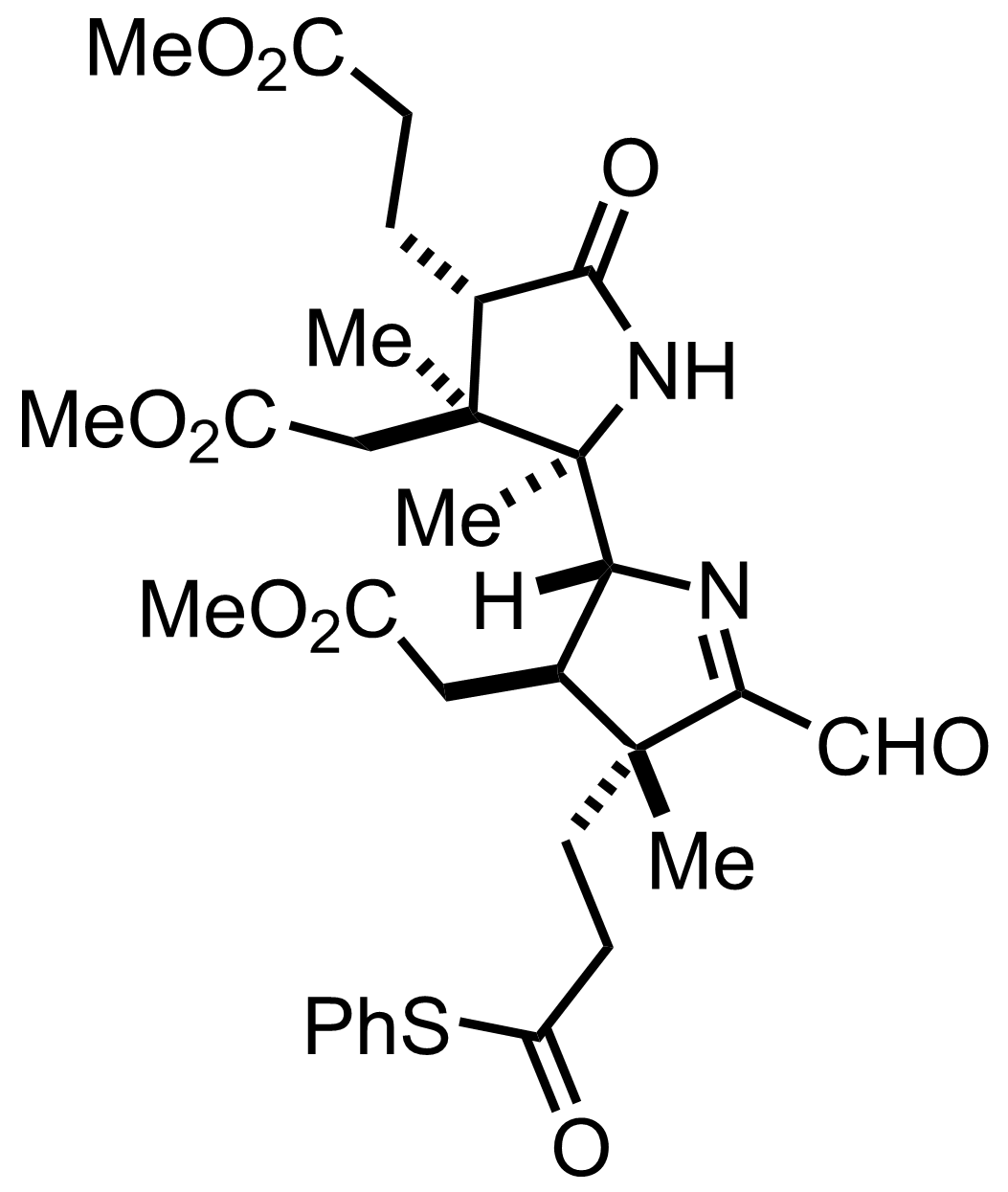
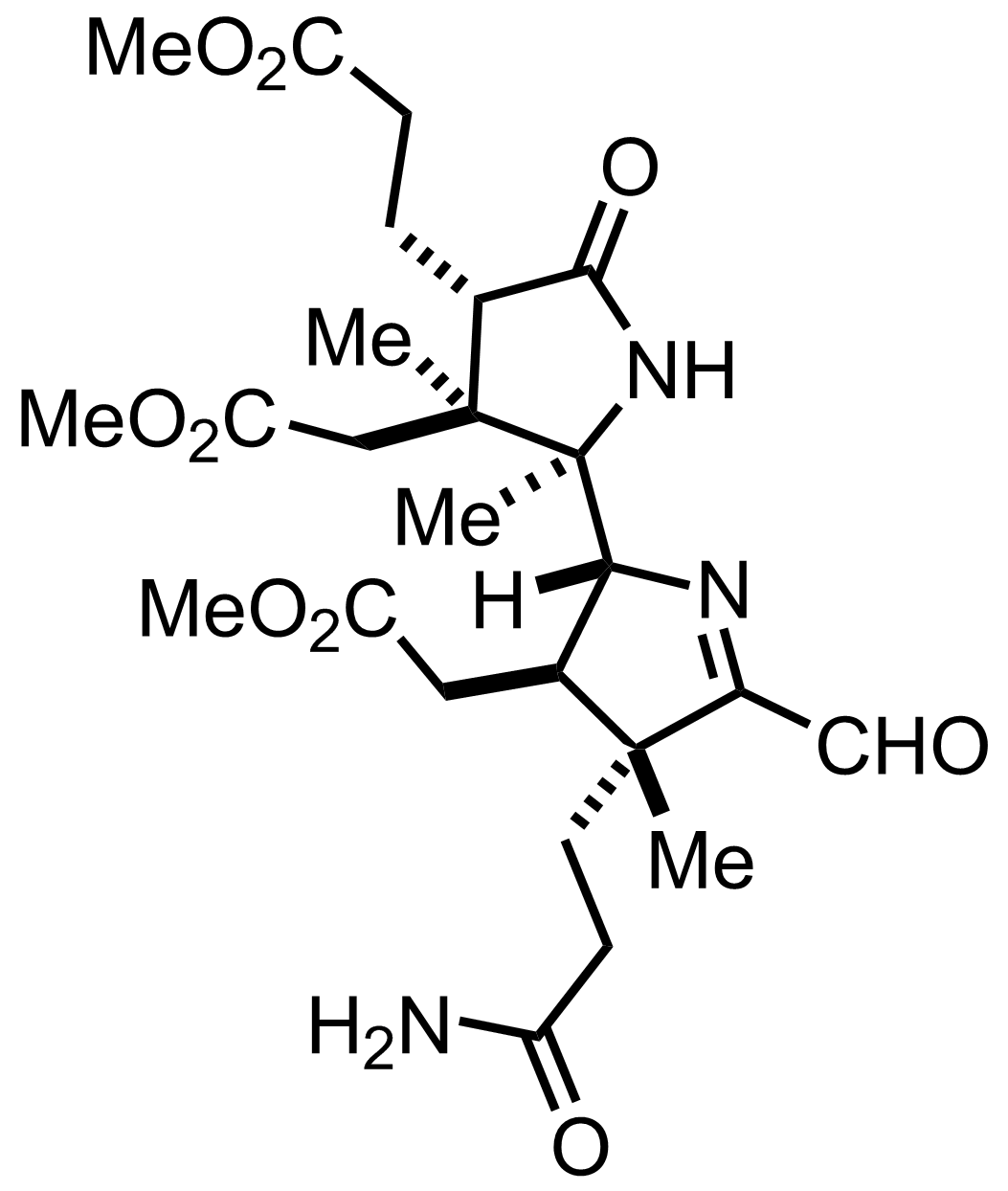
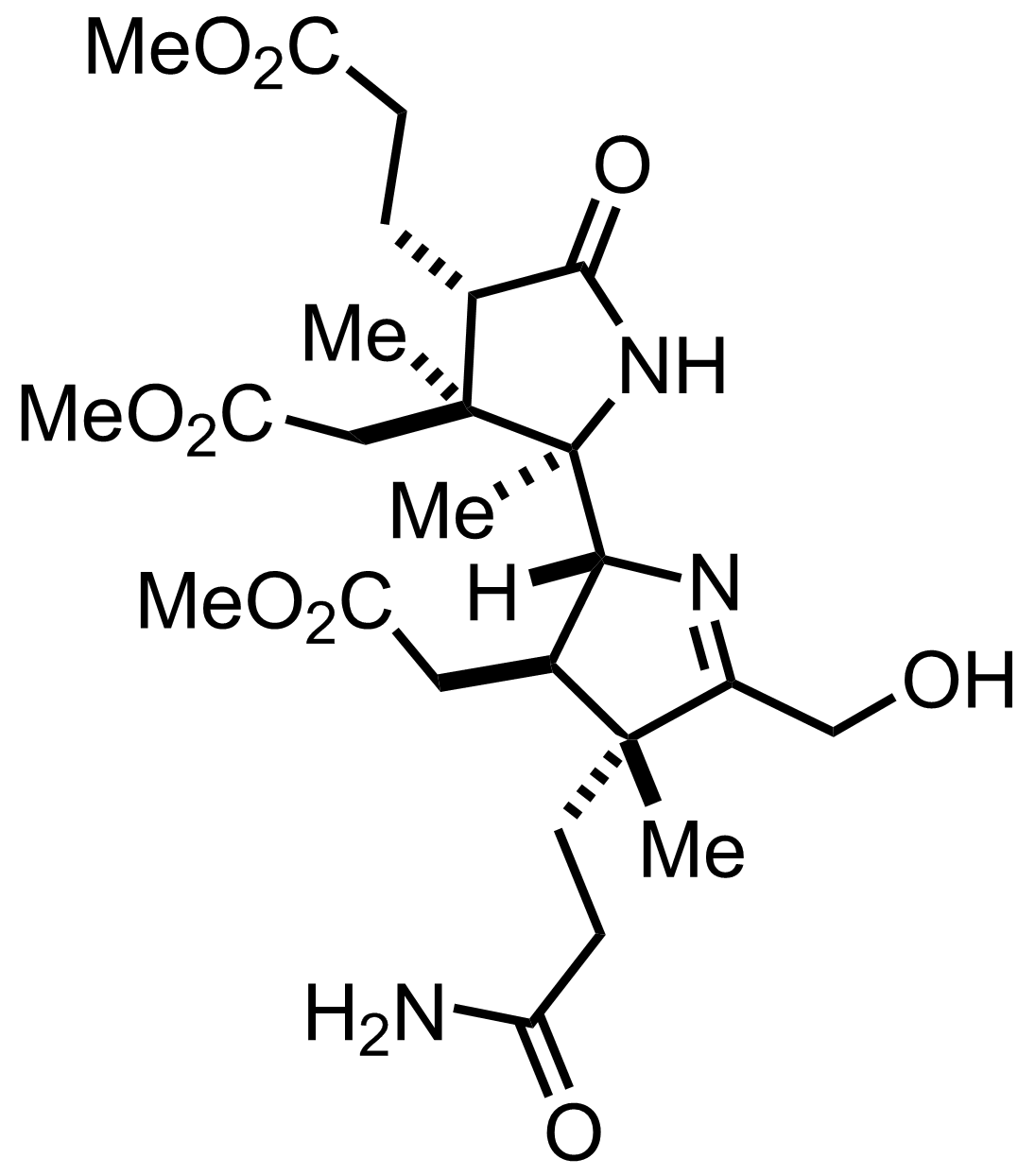
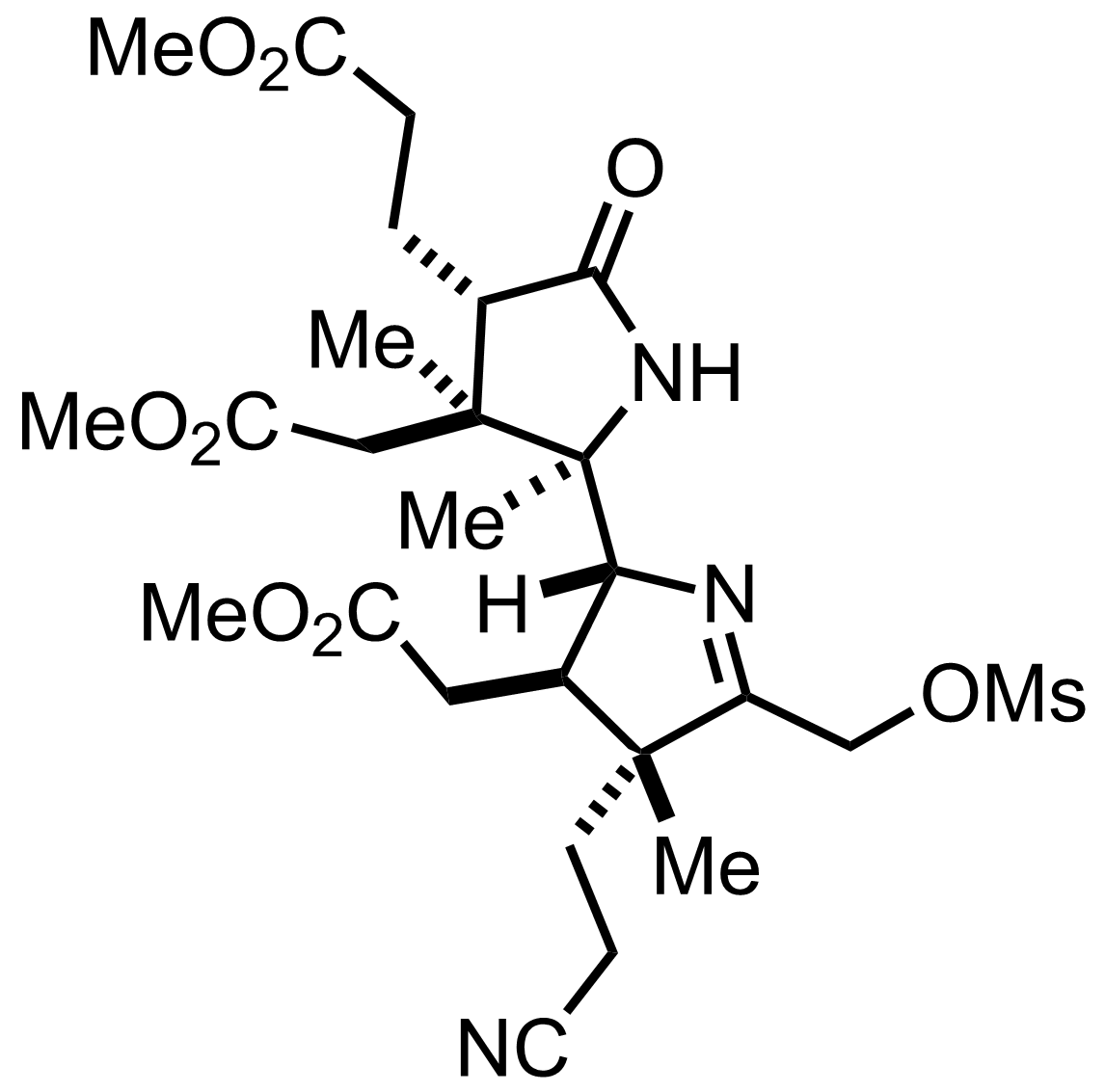
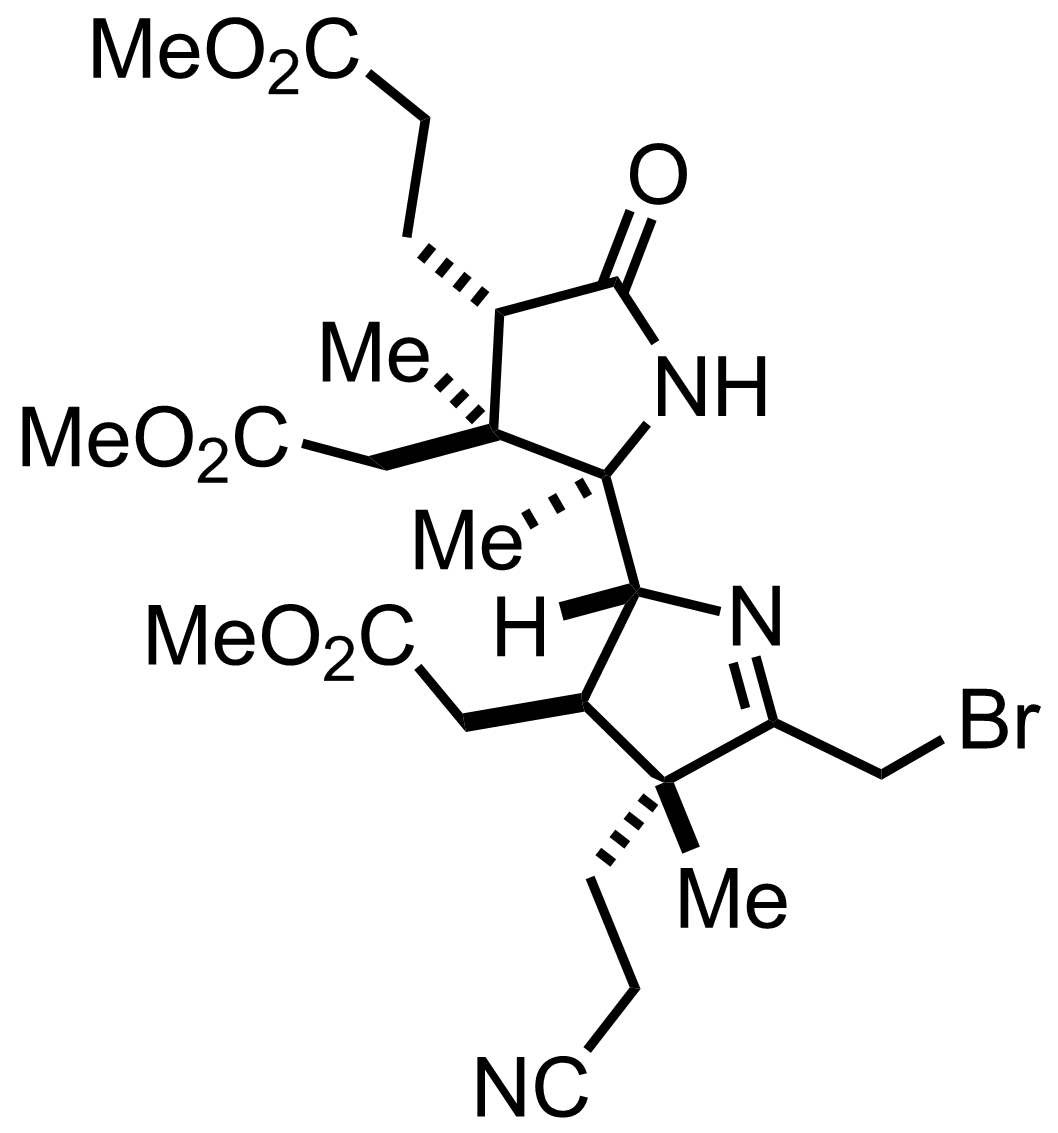
 +
+



(CH2OH)2,
TsOH
PhH

Et3O+ BF4-

MeOH

PhMe
Reflux


90%
""When subjected to acid treatment under carefully controlled condition""

""further treated with acid""

NH2OH

NaNO2
AcOH

HIO4,
O3
MeOH

CH2N2
Et2O


MsCl,
Et3N

HIO4,
O3
EtOAc, H2O

CH2N2
Et2O

Polystyrenesulfonic acid
MeOH
170 °C
See the Beckmann Rearrangement
"Also isolated was the C3-epimer which was equilibrated to the desired product."

HCl,
PhSH
MeOH

O3

NH3

NaBH4

Ms2O


Part 7 of 7
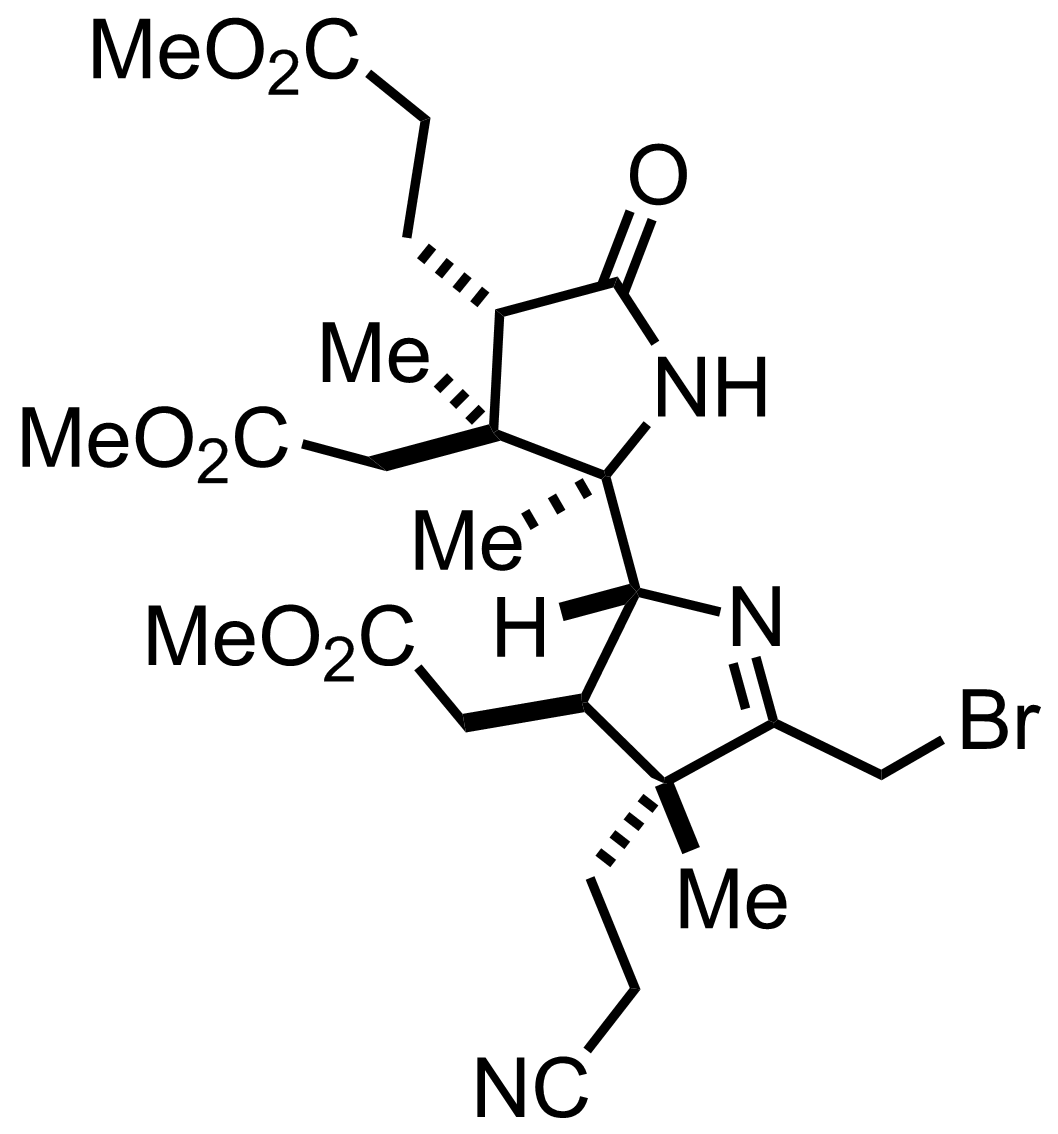 +
+
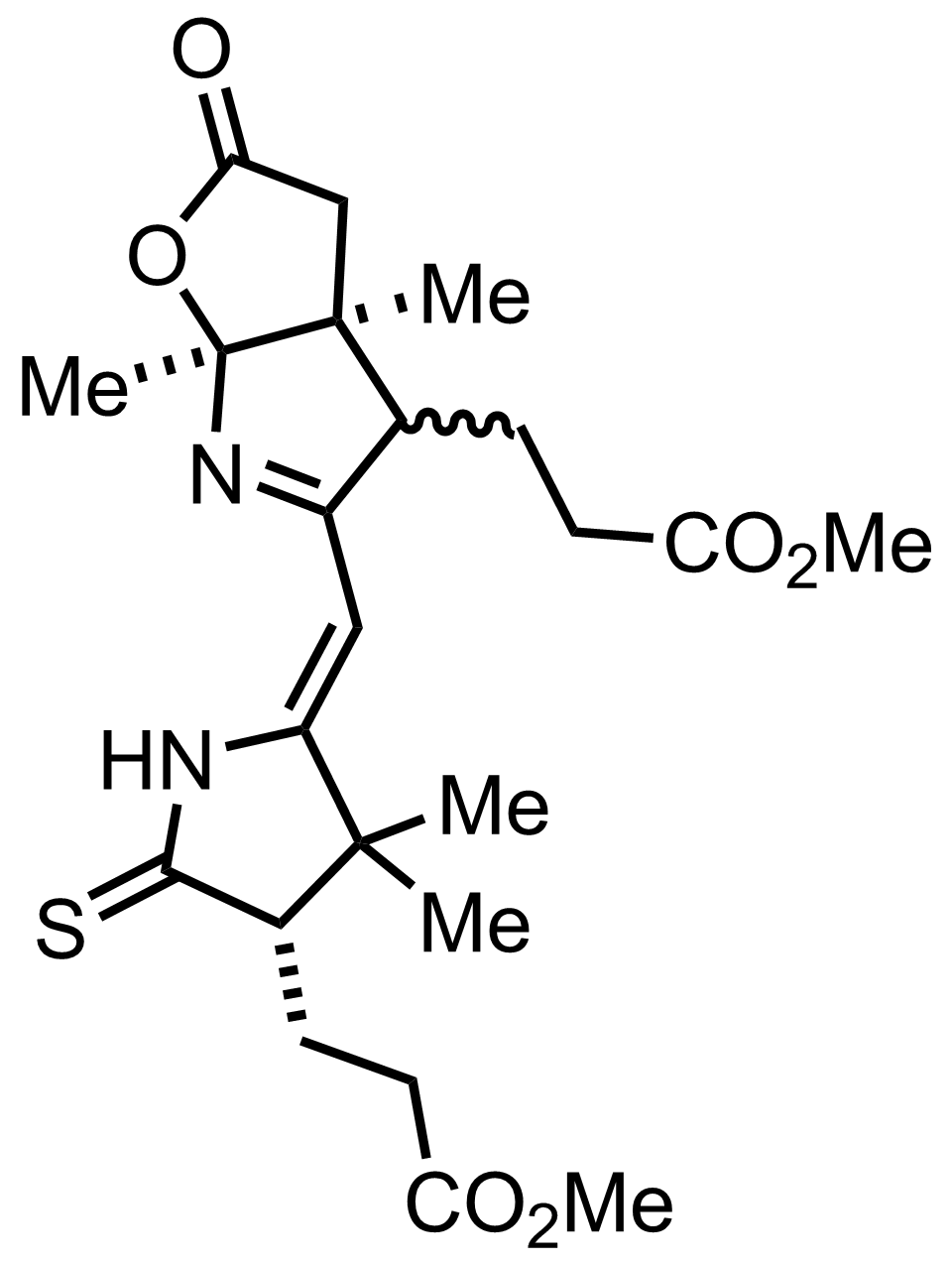
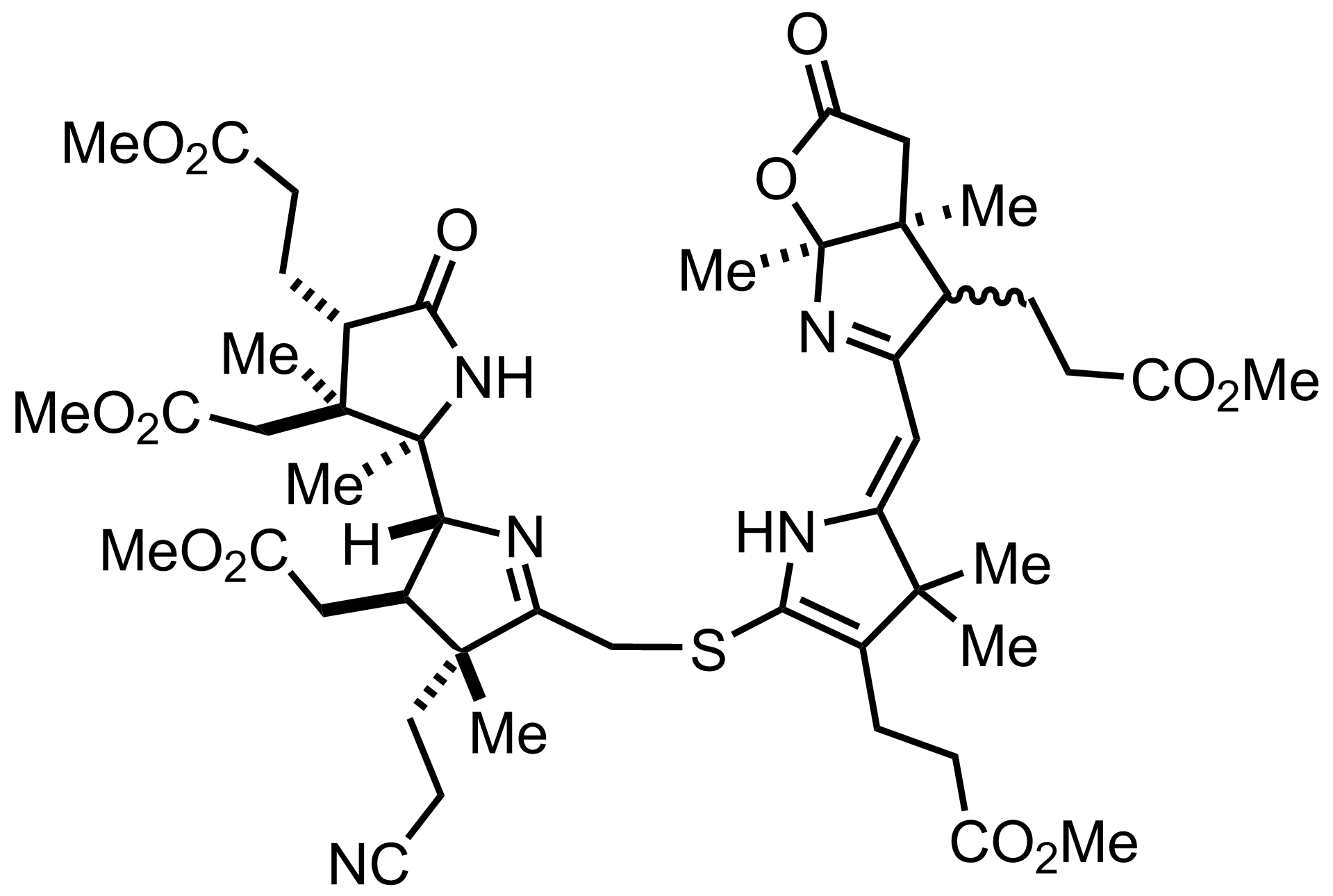
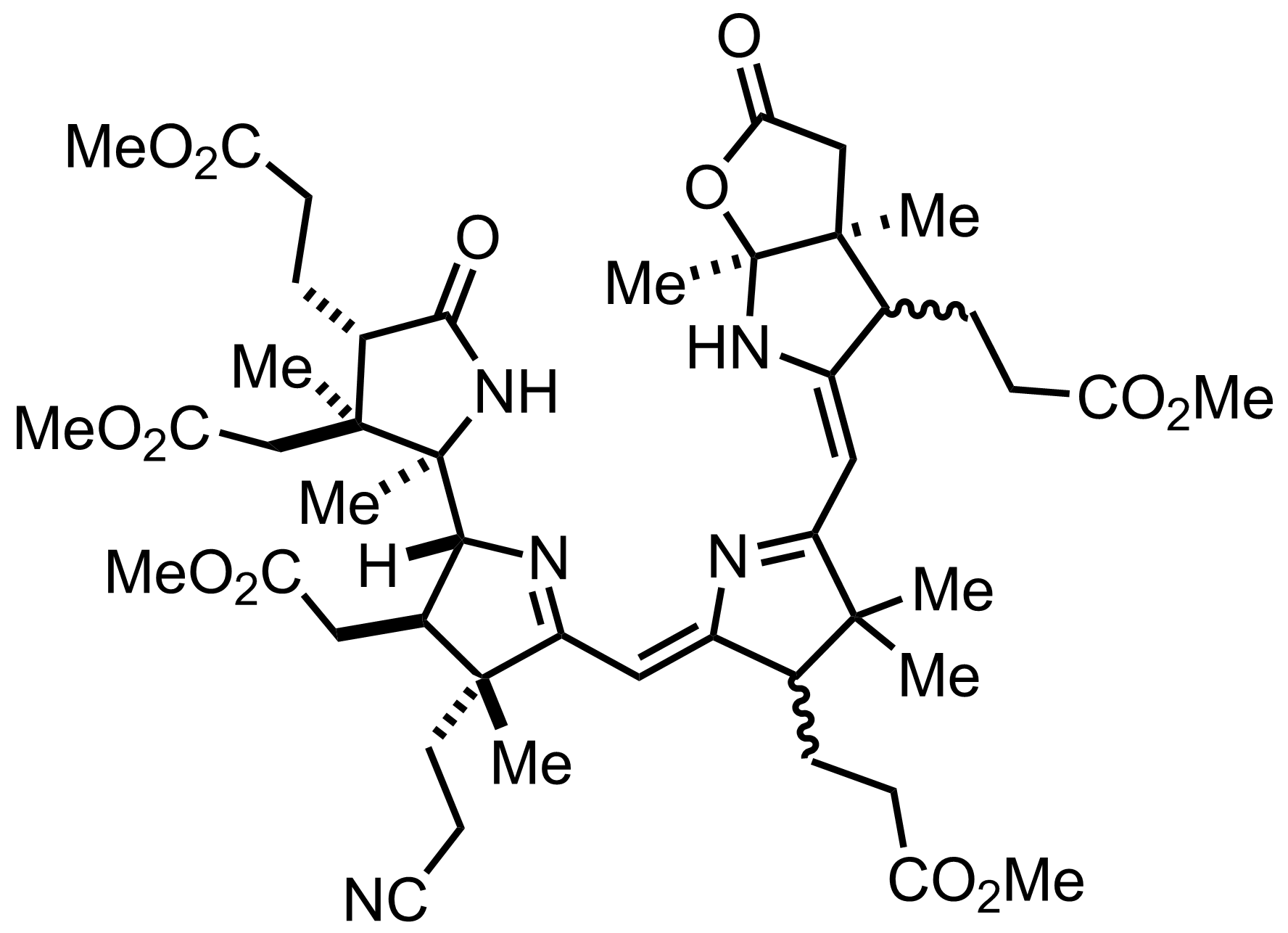
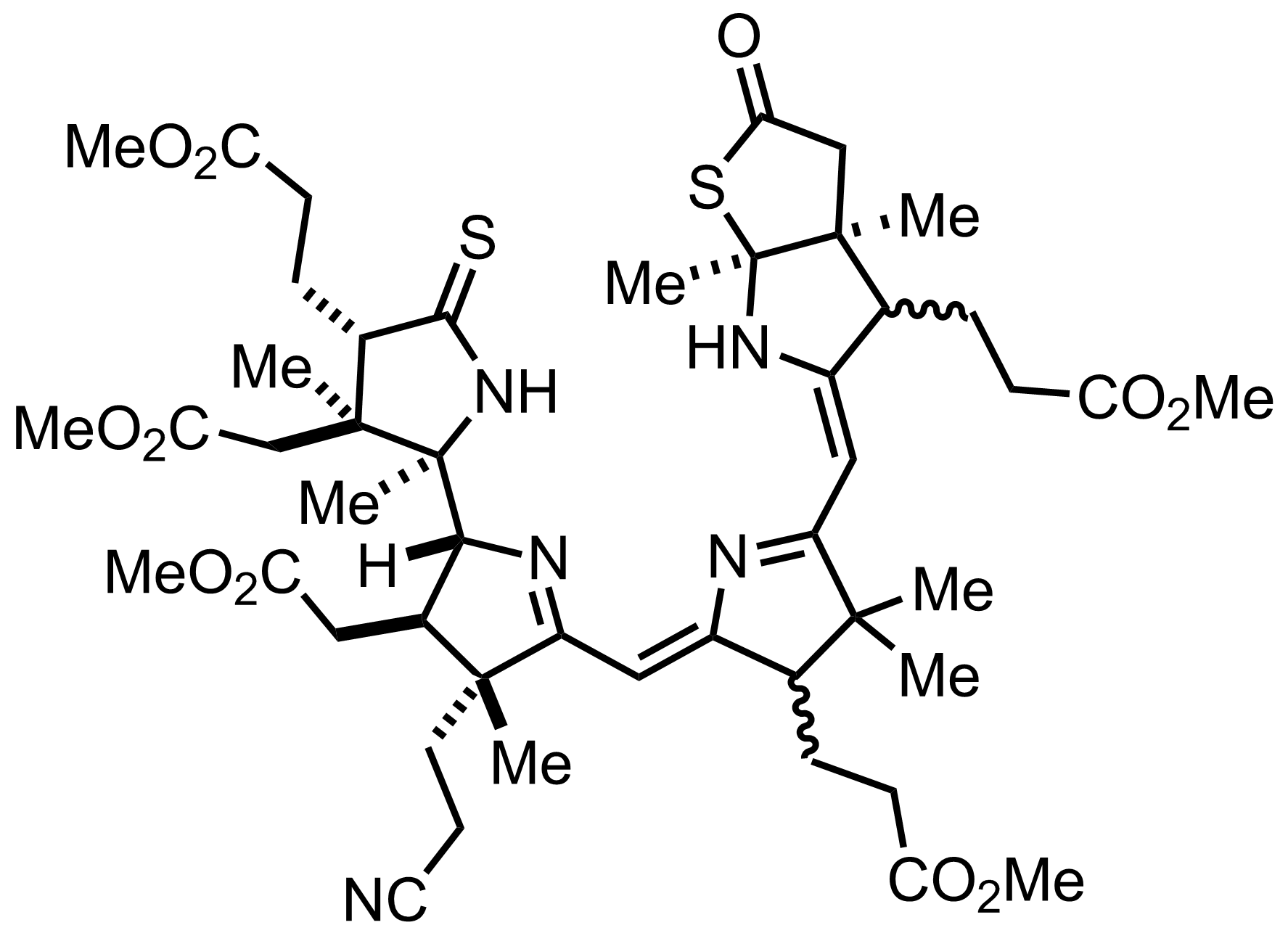
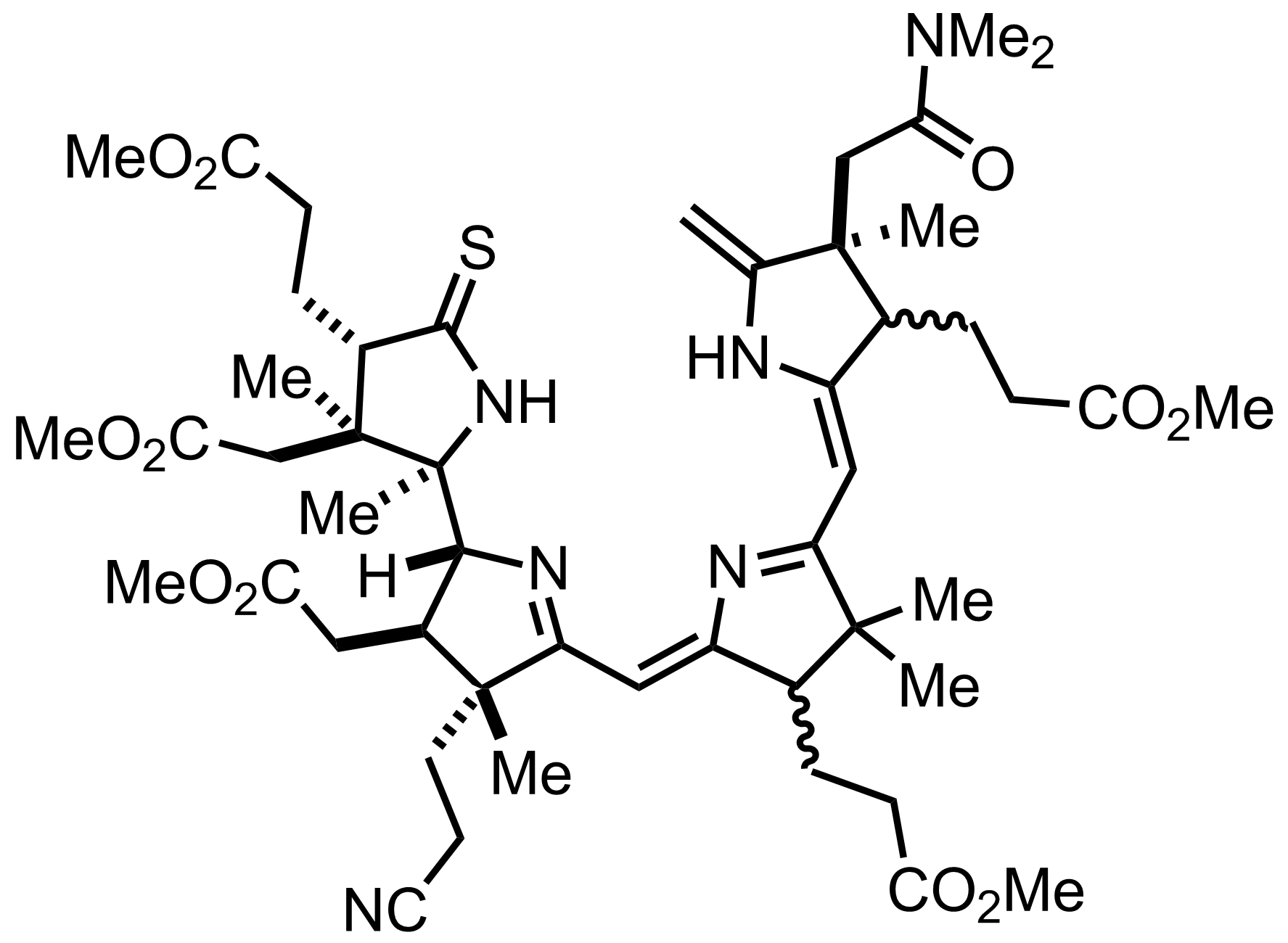
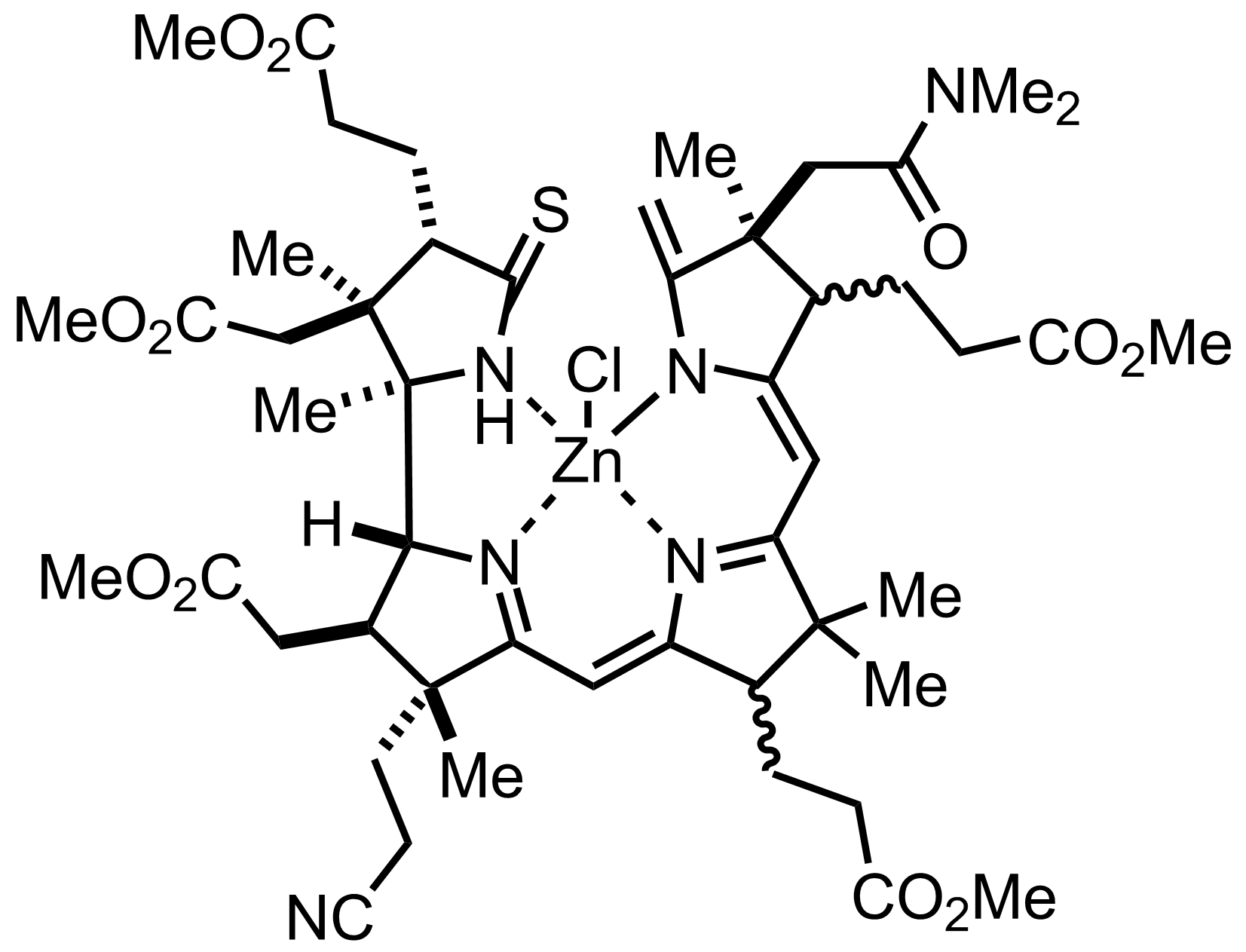
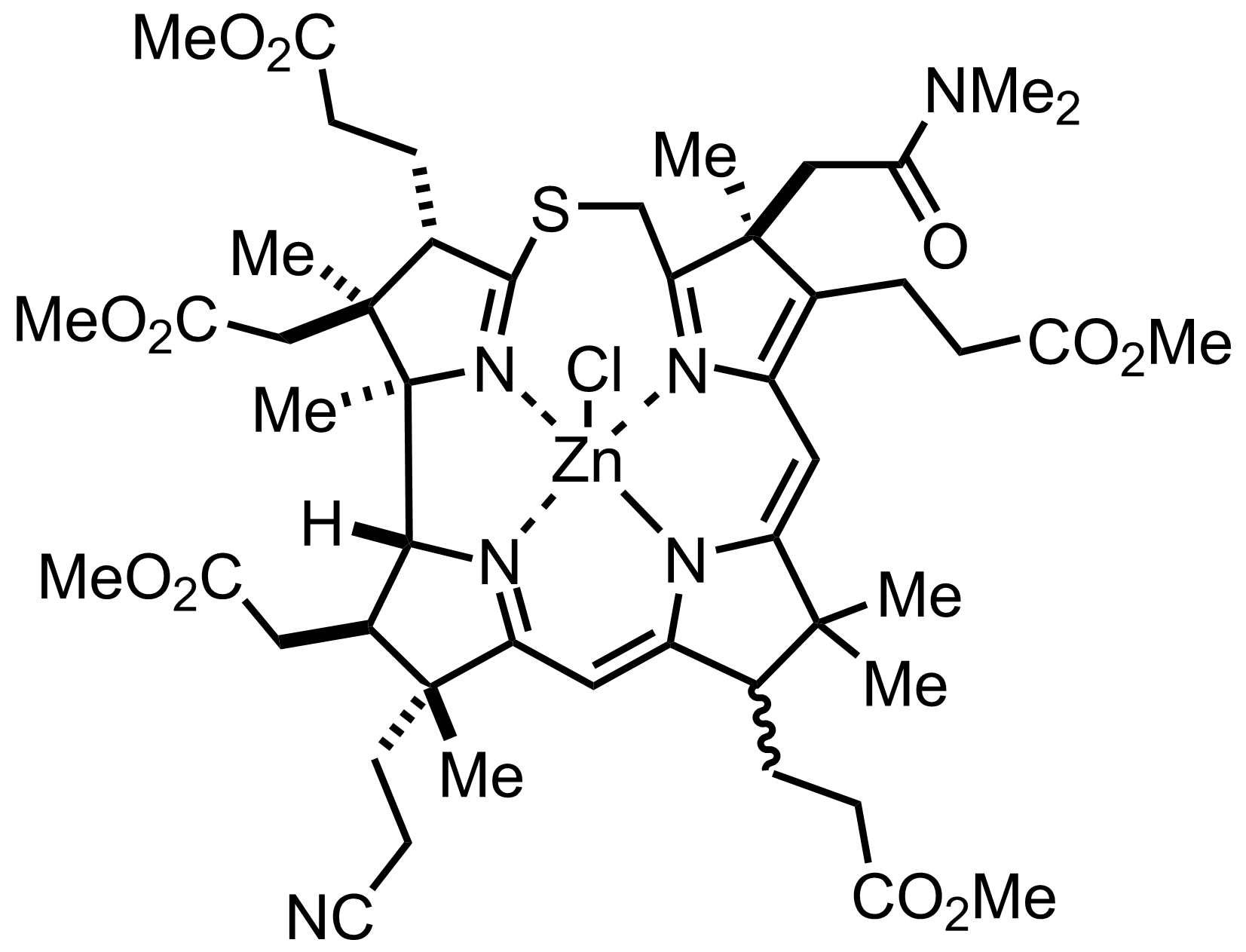
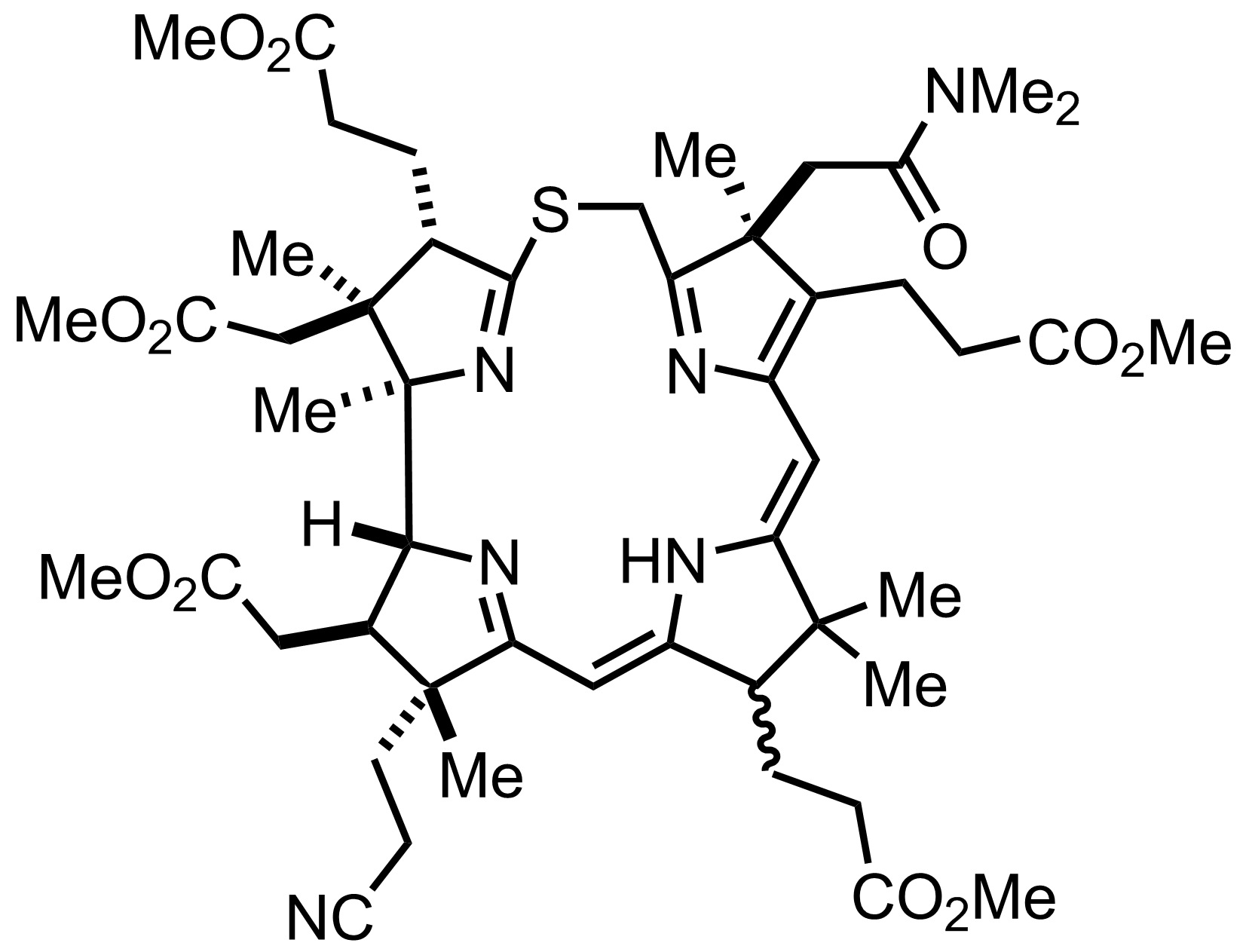
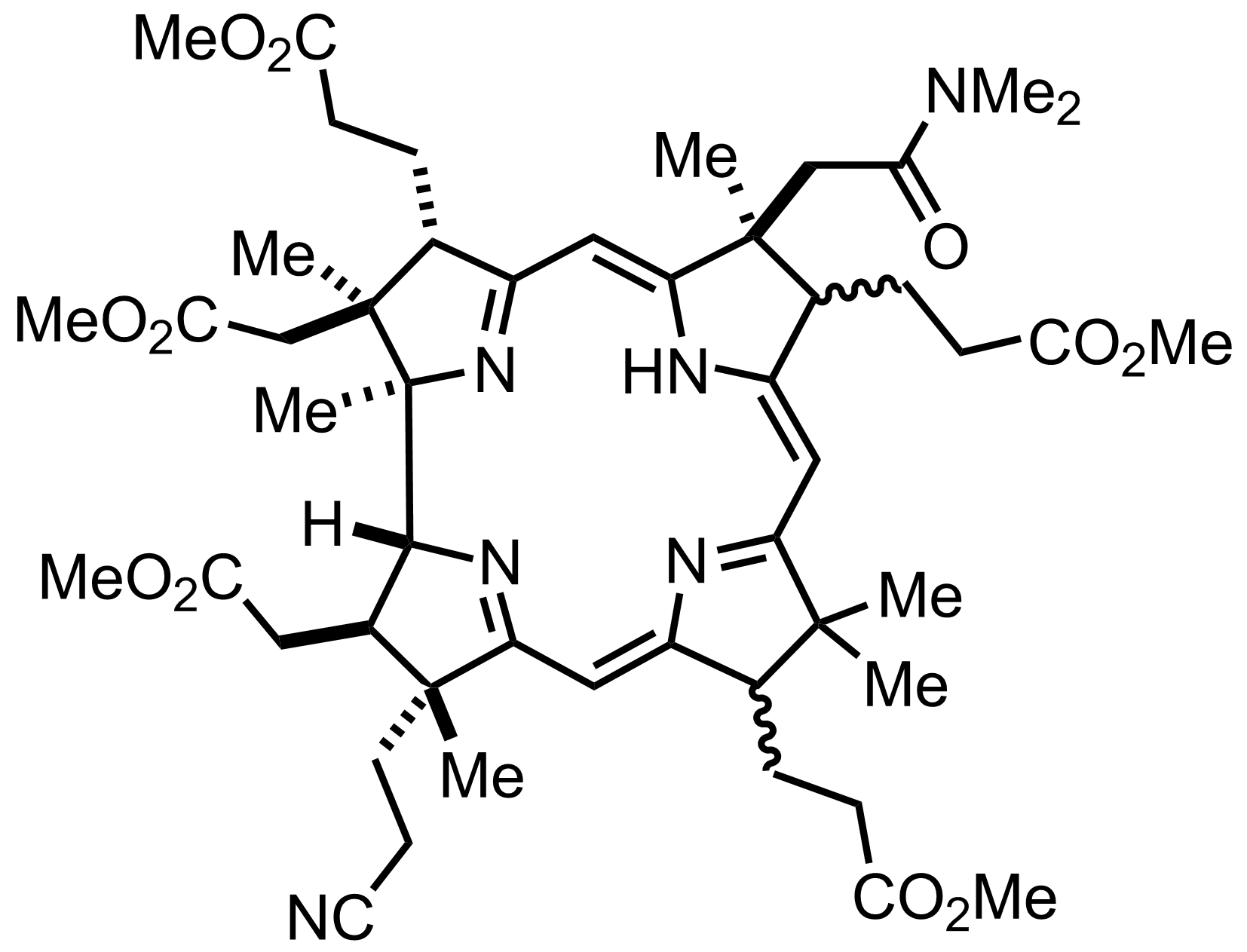
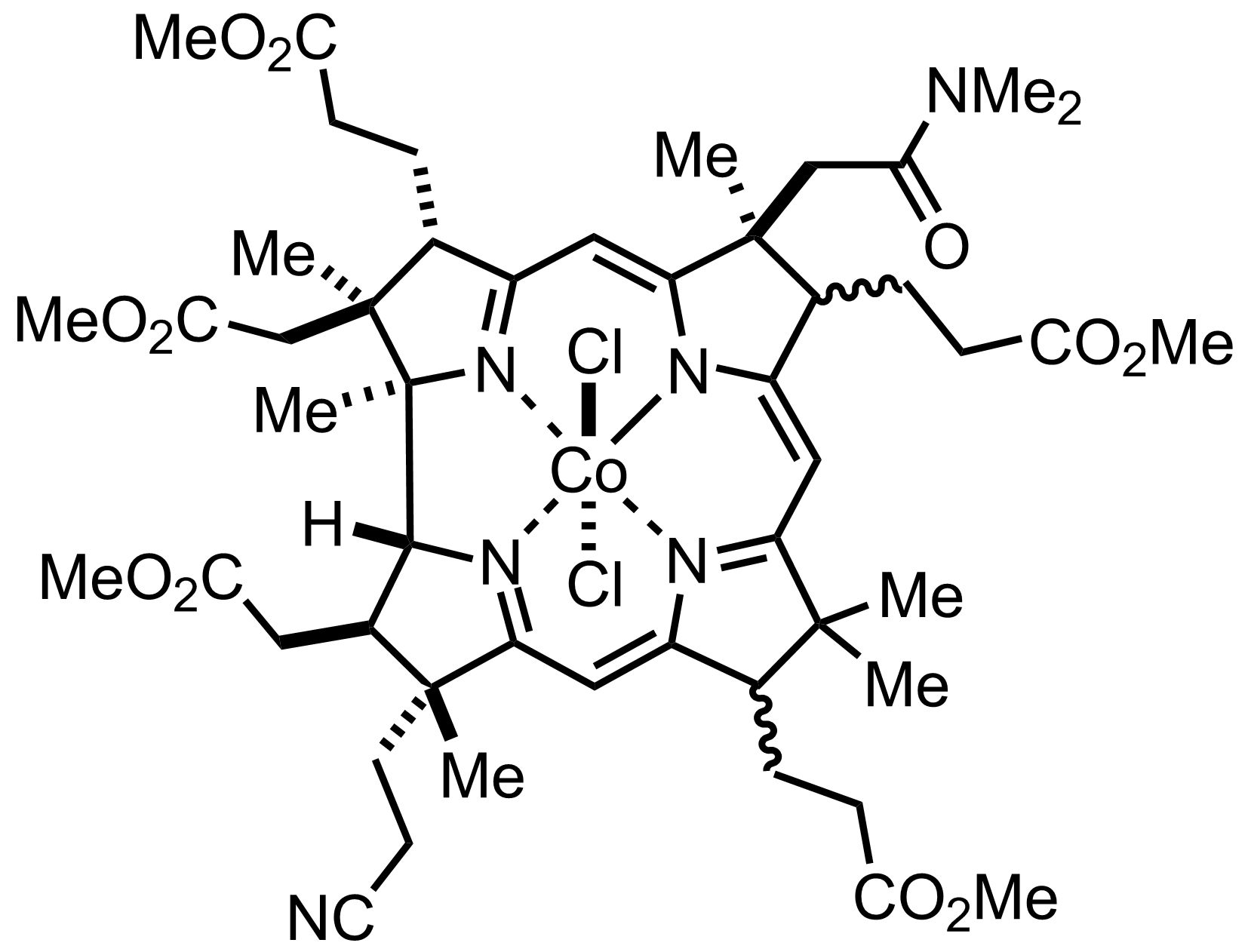
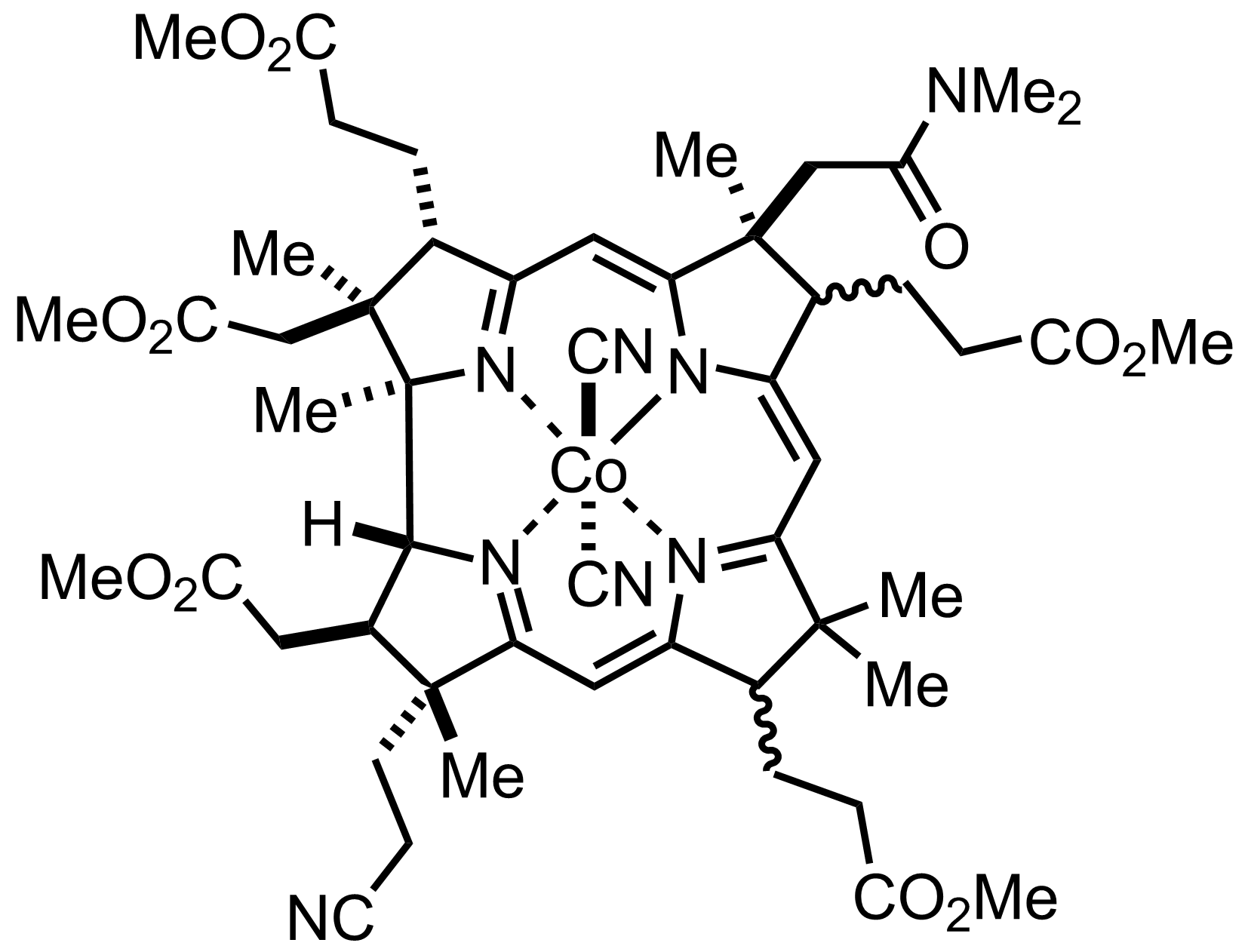
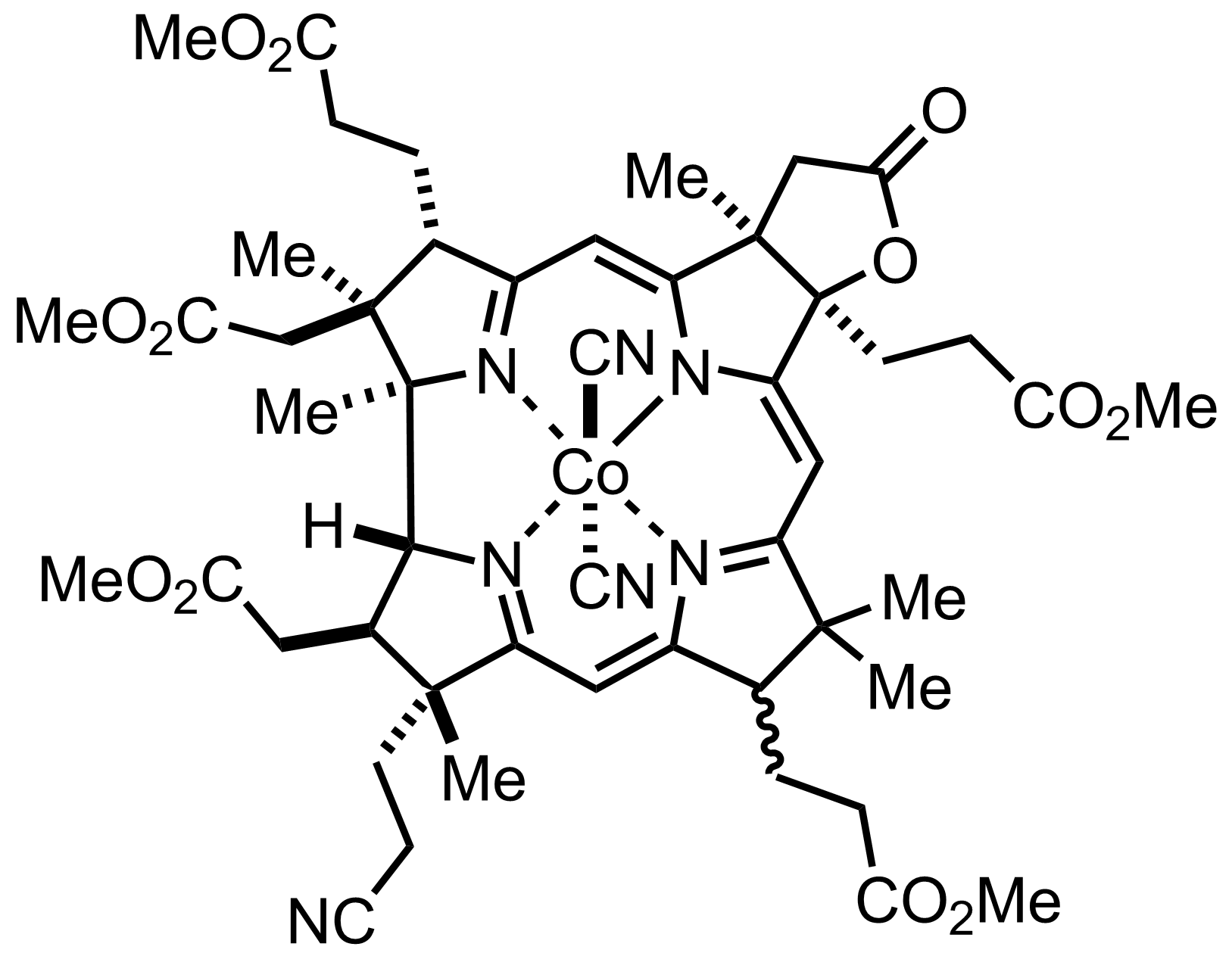
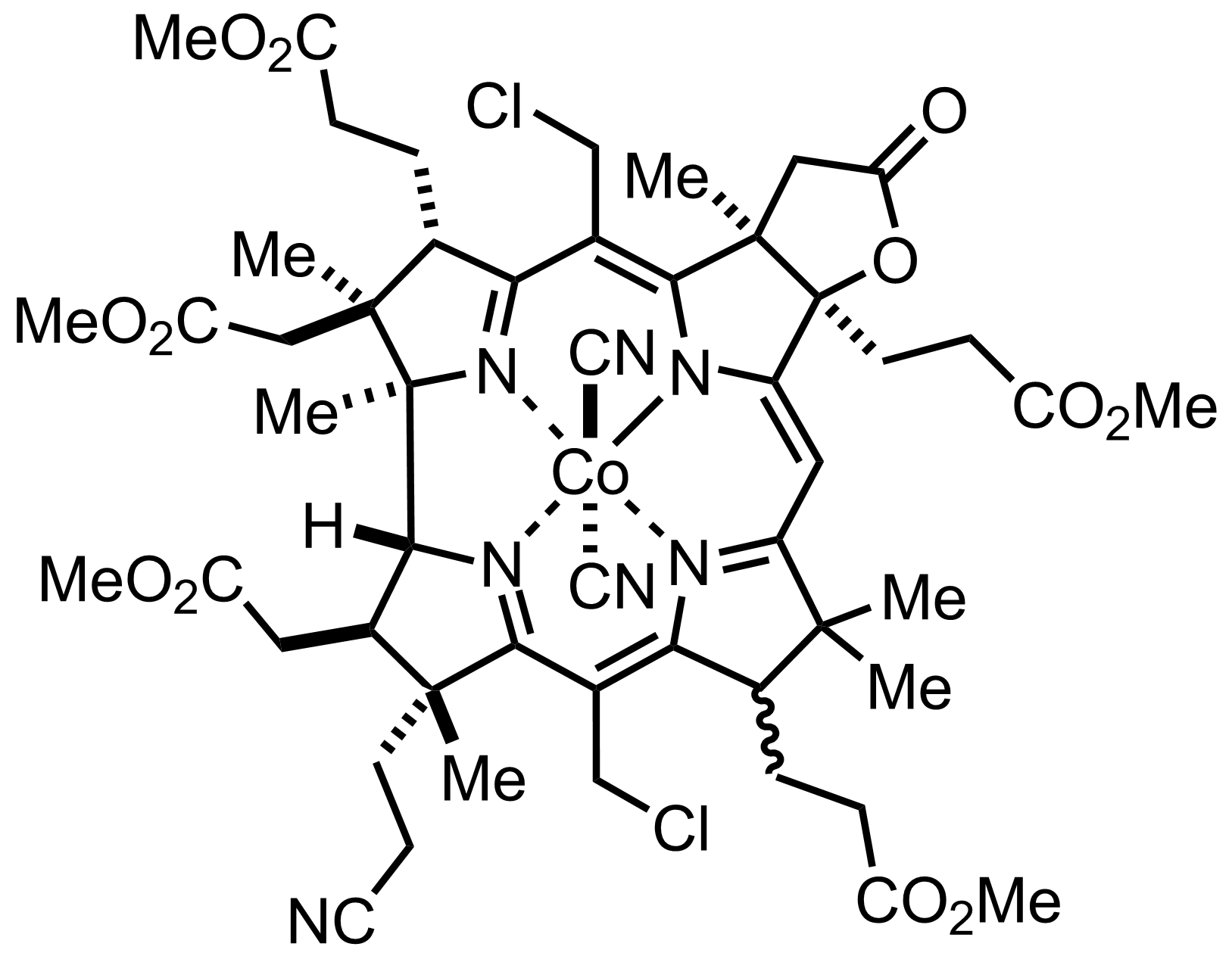
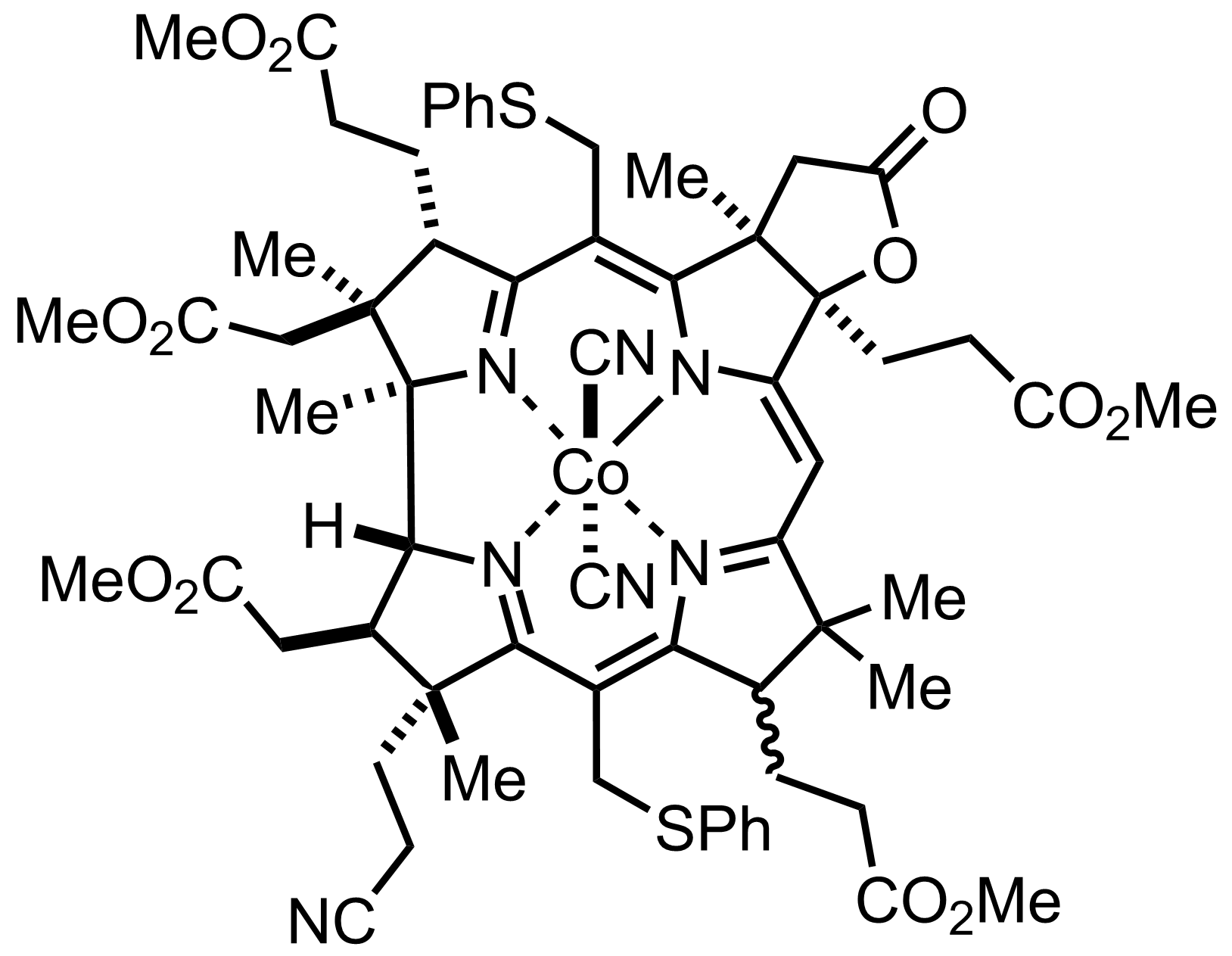
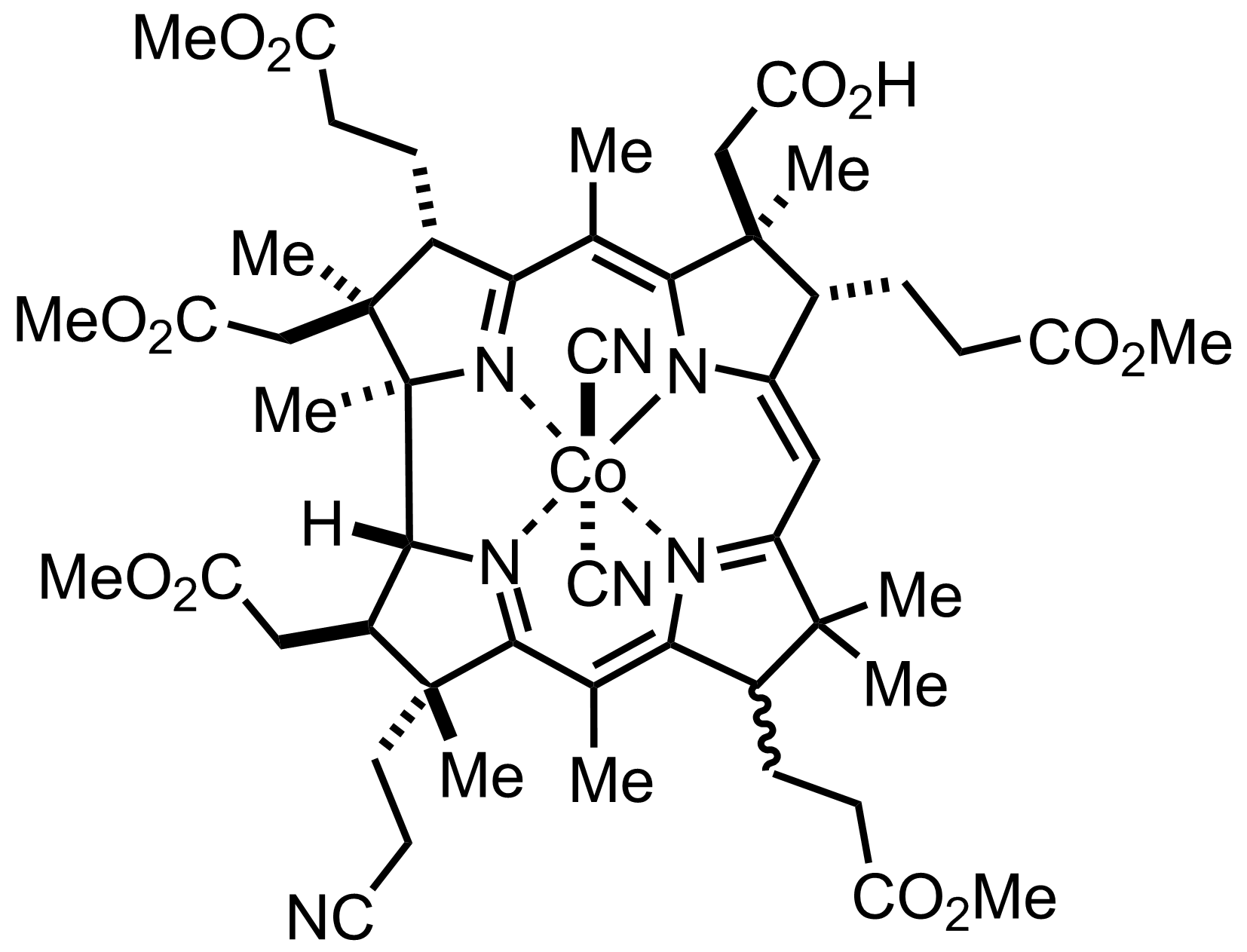
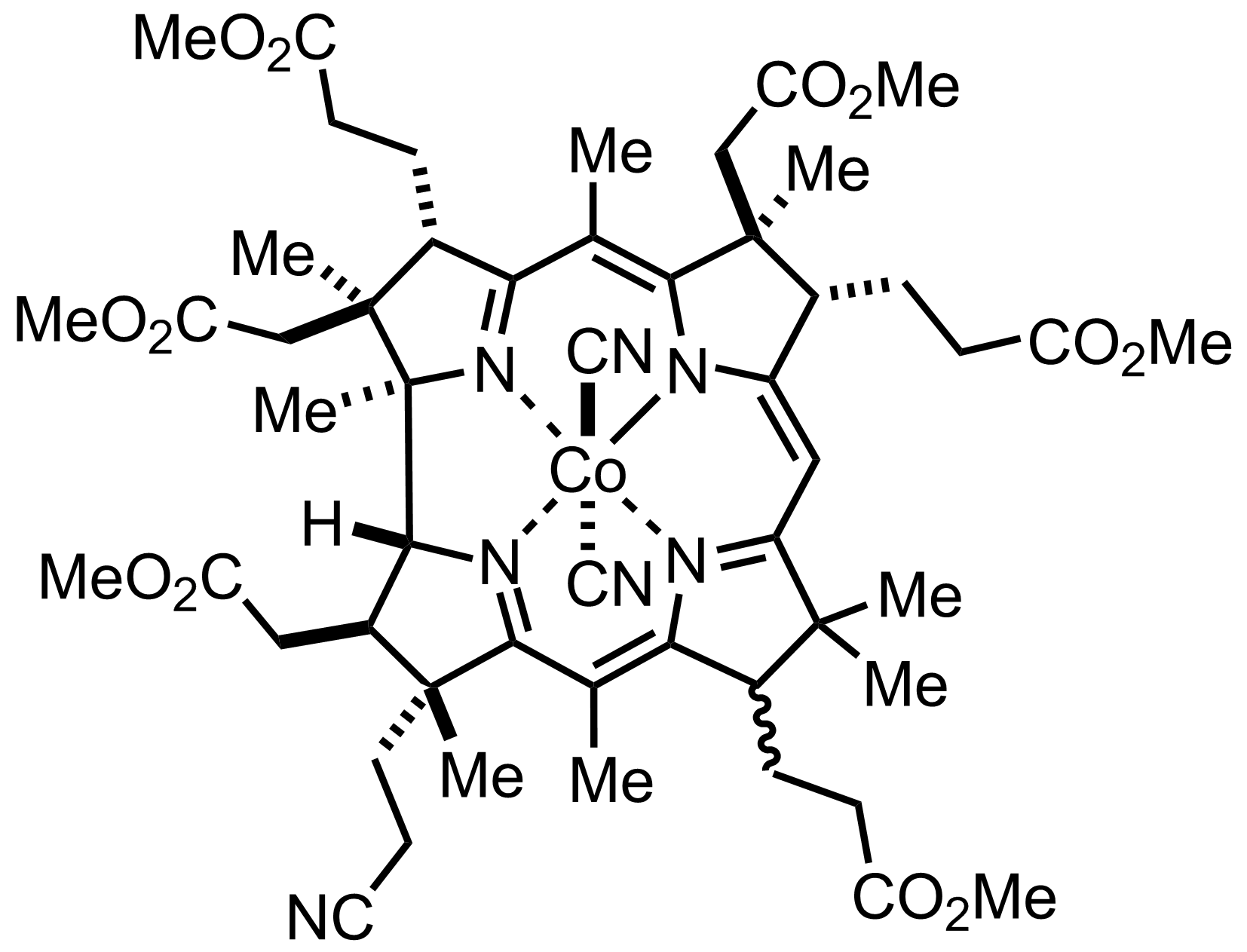
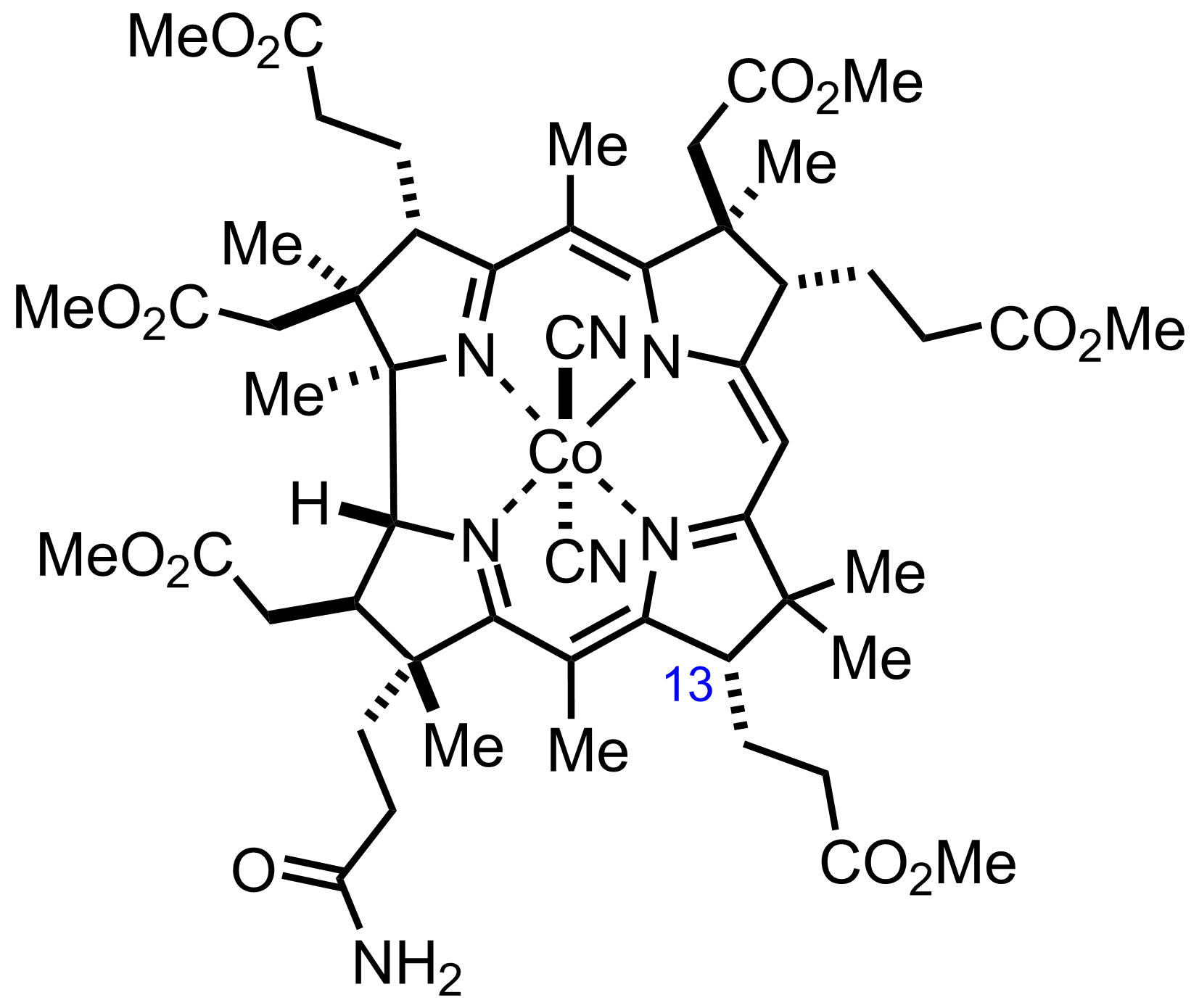
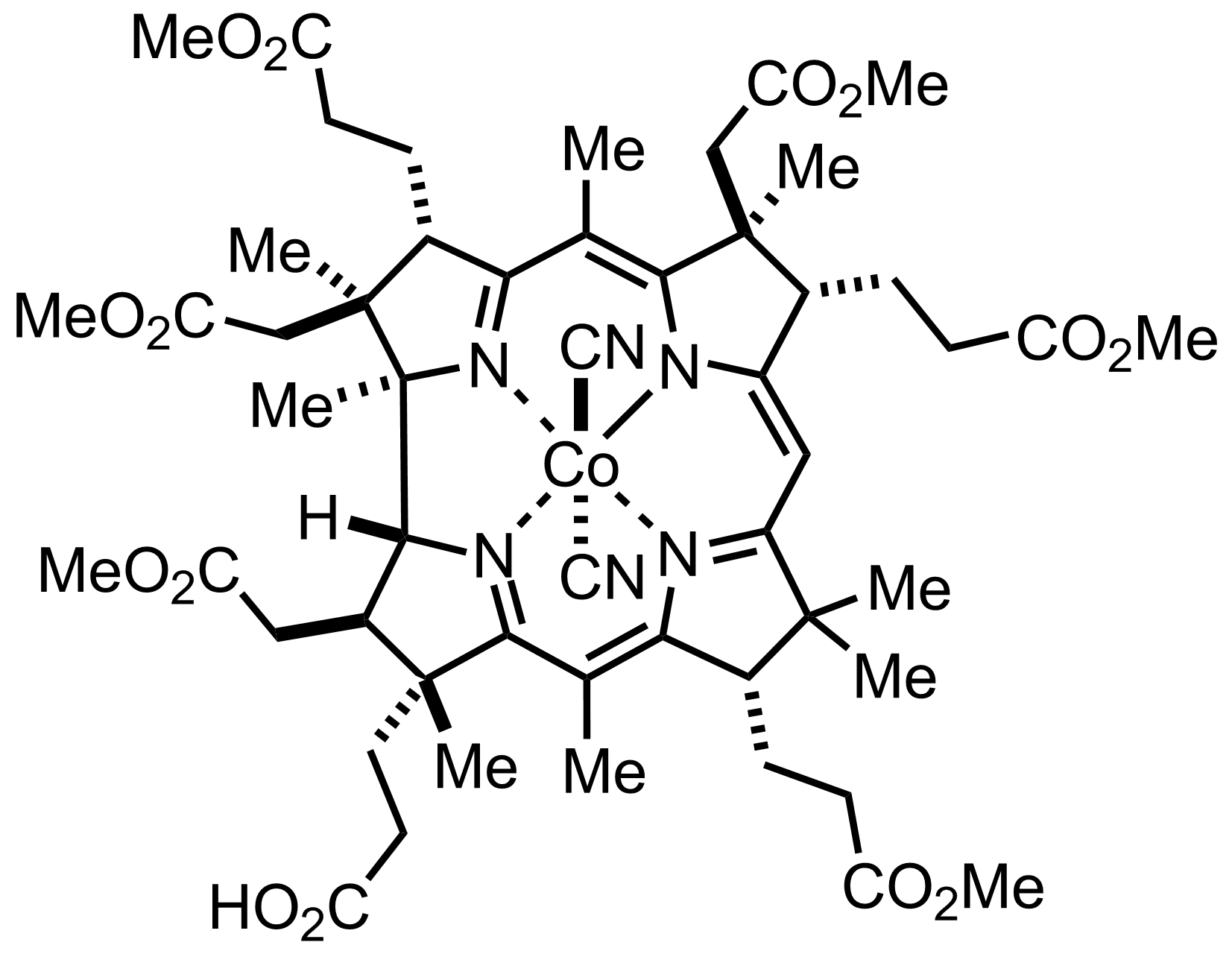
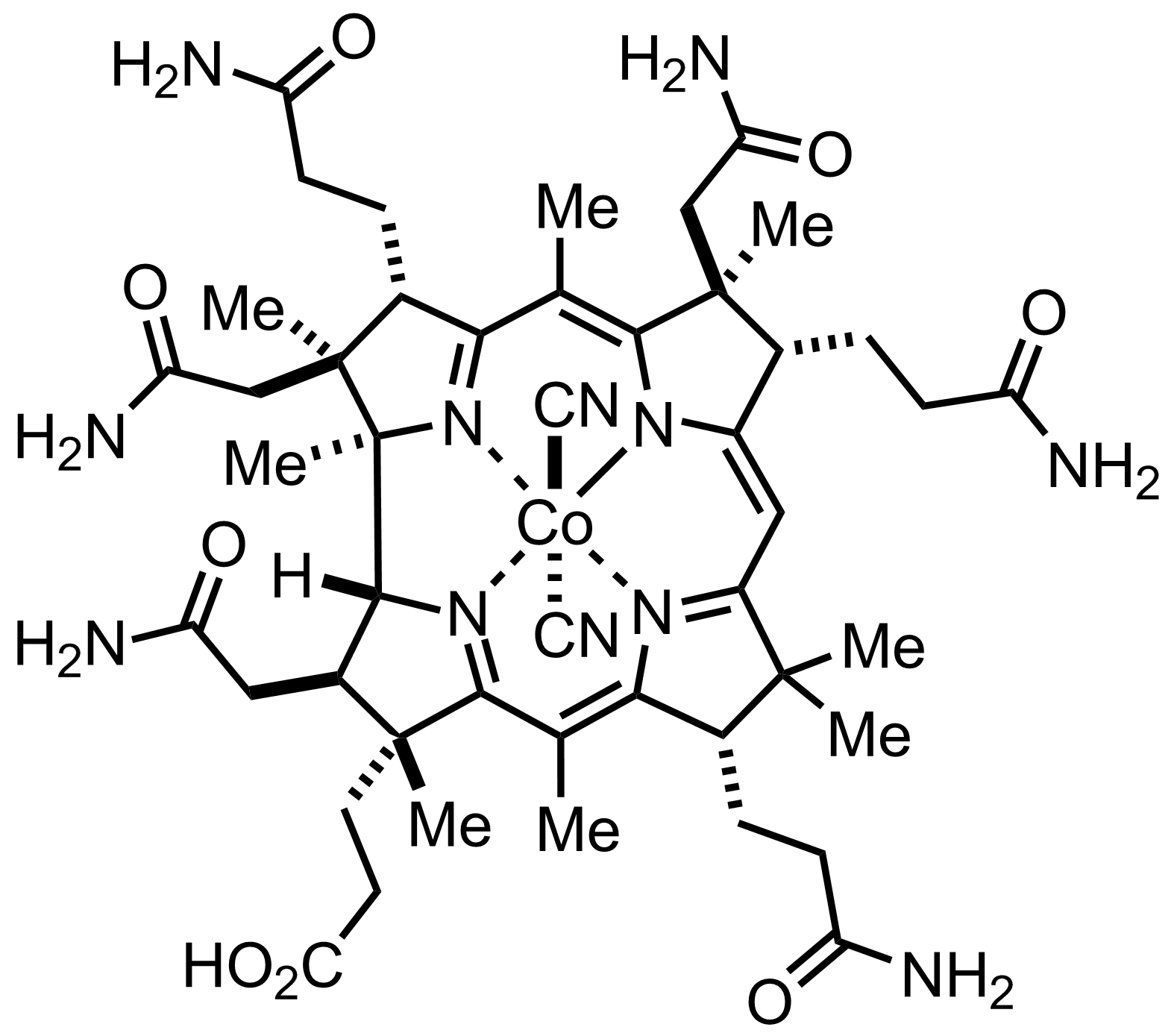
 +
+

KOt-Bu
100%


P2S5,
4-Methylpyridine
PhMe

Me2NH

ZnCl2

I2
MeOH

""it was a relatively easy matter to remove the zinc by treatment with acid""


CoCl2
THF

NaCN

I2
AcOH


PhSH

Ni (Raney)

CH2N2
Et2O

H2SO4
H2O
60 min
"Also isolated was the C13-epimer in an unfavorable 28:72 ratio."

N2O4,
NaOAc
CCl4
0 °C, 60 min, 70-80%

NH3,
NH4Cl
Ethylene Glycol
75 °C, 10 h, 100%

Remaining...
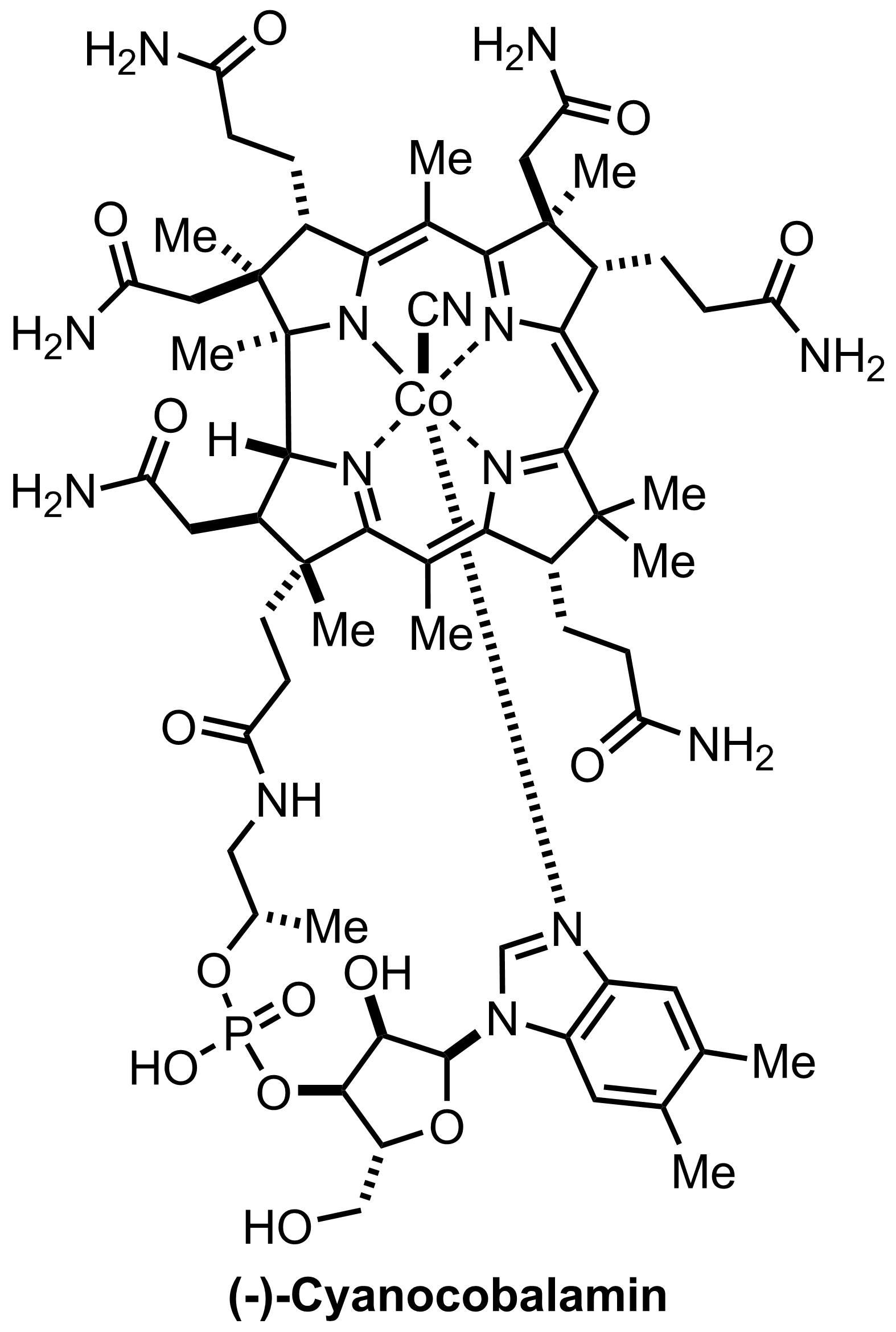
(1 step)
"See: Helv. Chim. Acta 1960 , 43, 704."

