Synthesis of Ingenol
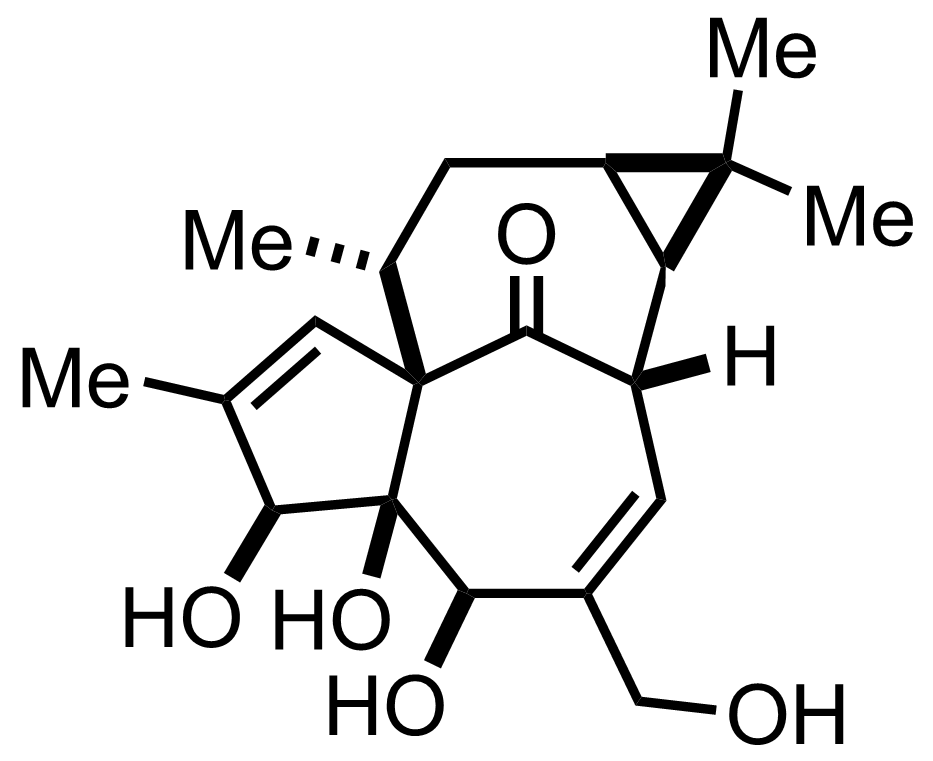
C20H28O5
| Principal investigator | Jeffrey D. Winkler |
|---|---|
| Publication year | 2002 |
| Synthesis type | Total |
| Number of steps | 45 (linear) |
| References |
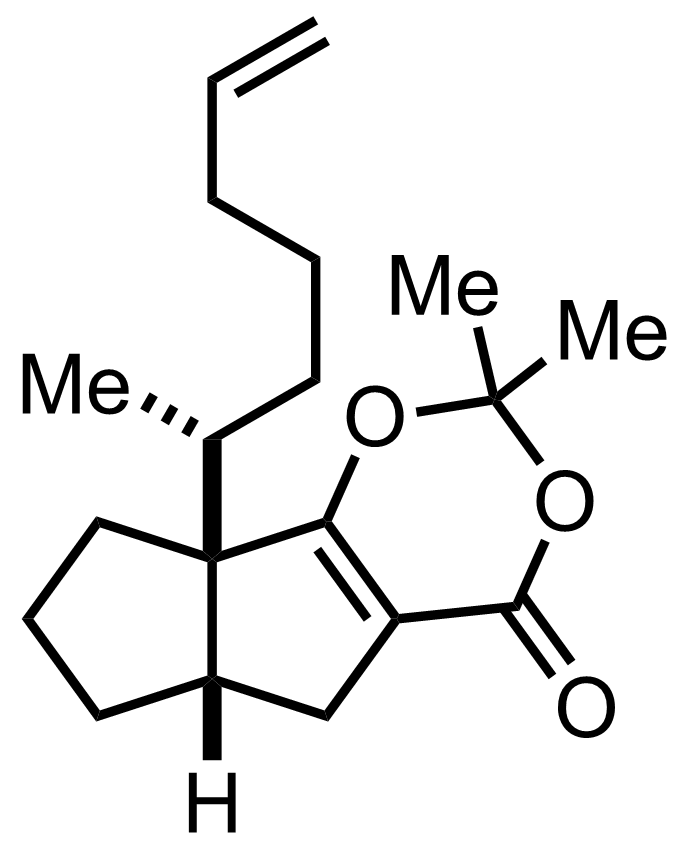
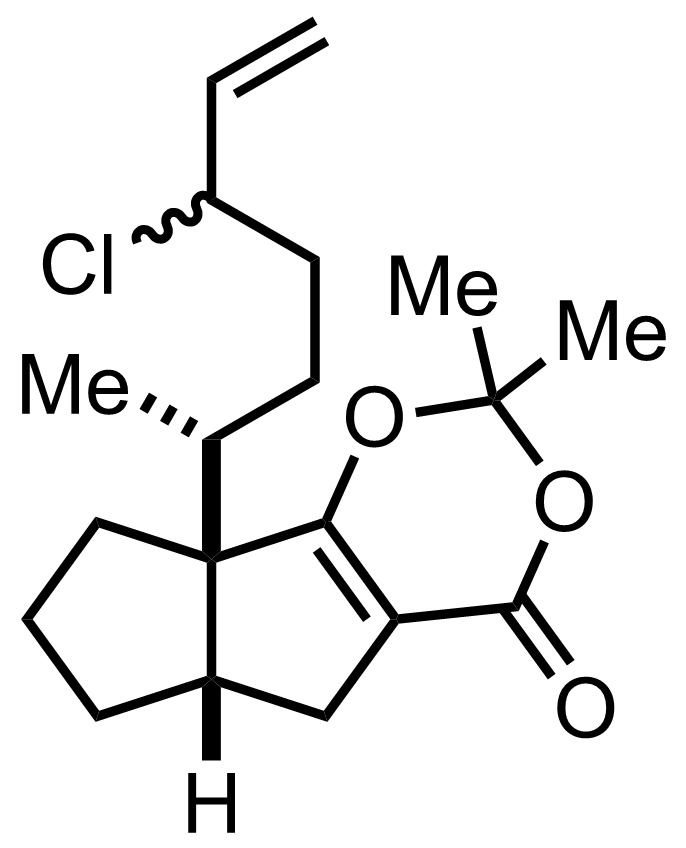
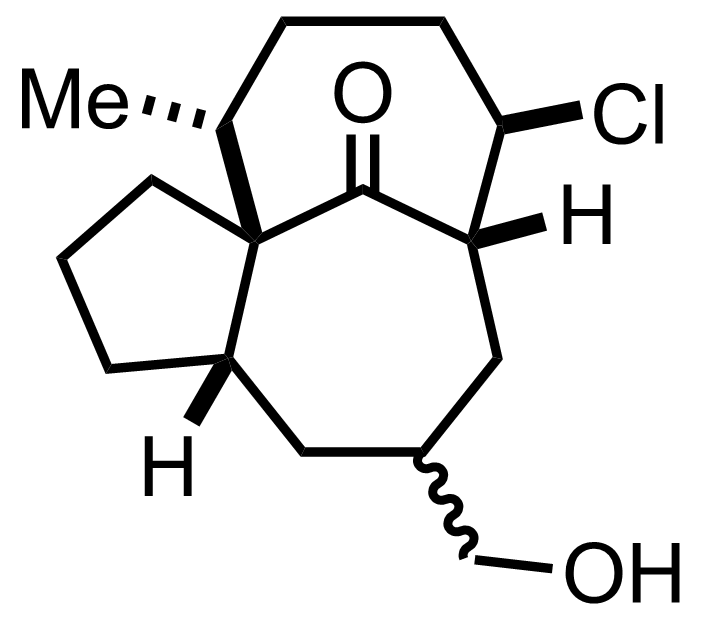
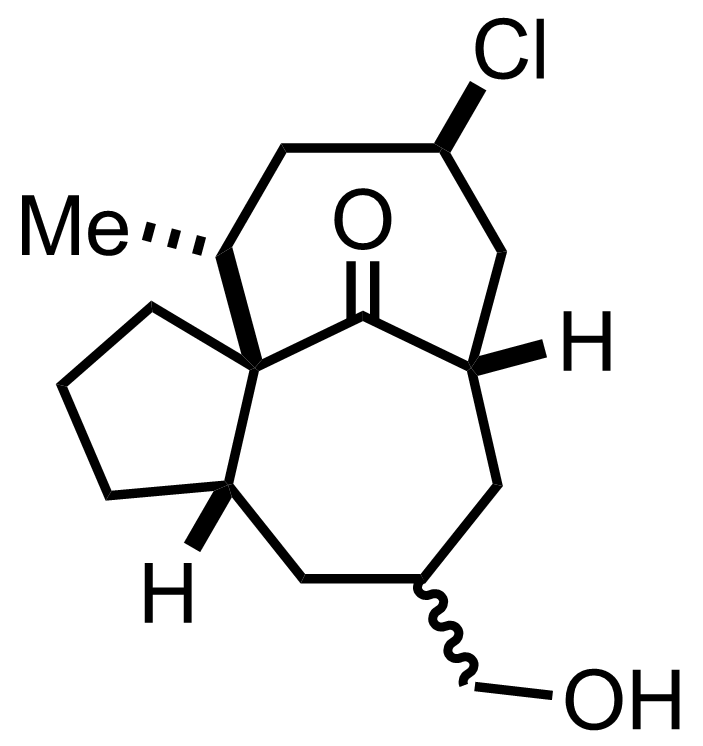
Part 1 of 1
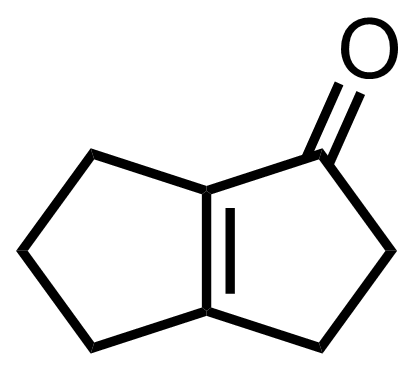 +
+
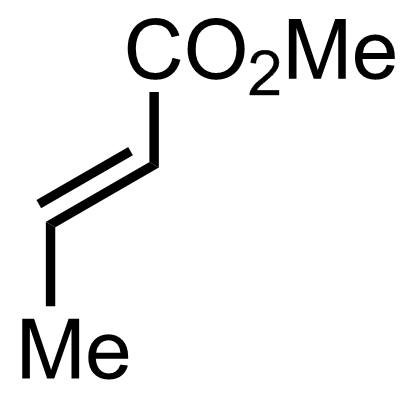
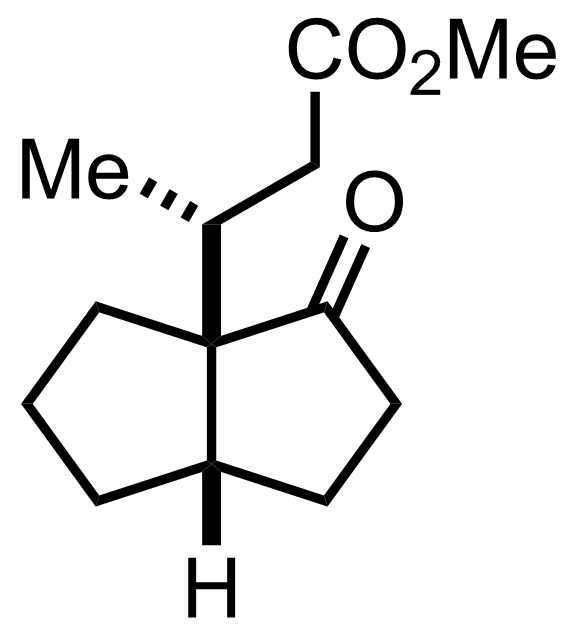




 +
+

 +
+

 +
+
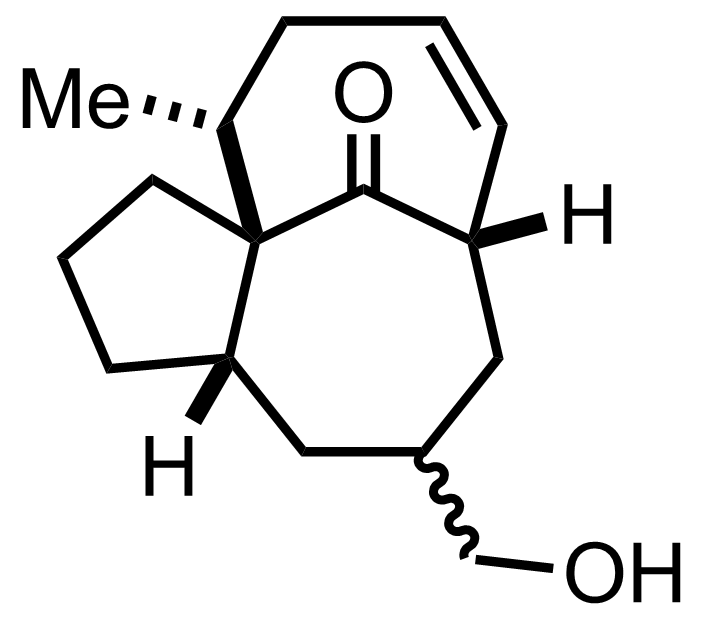

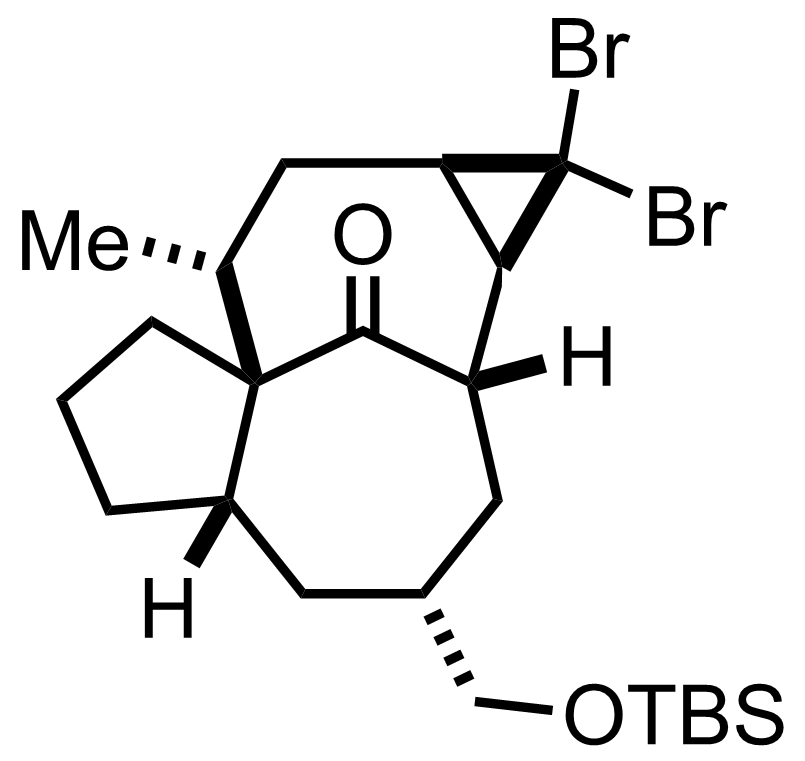
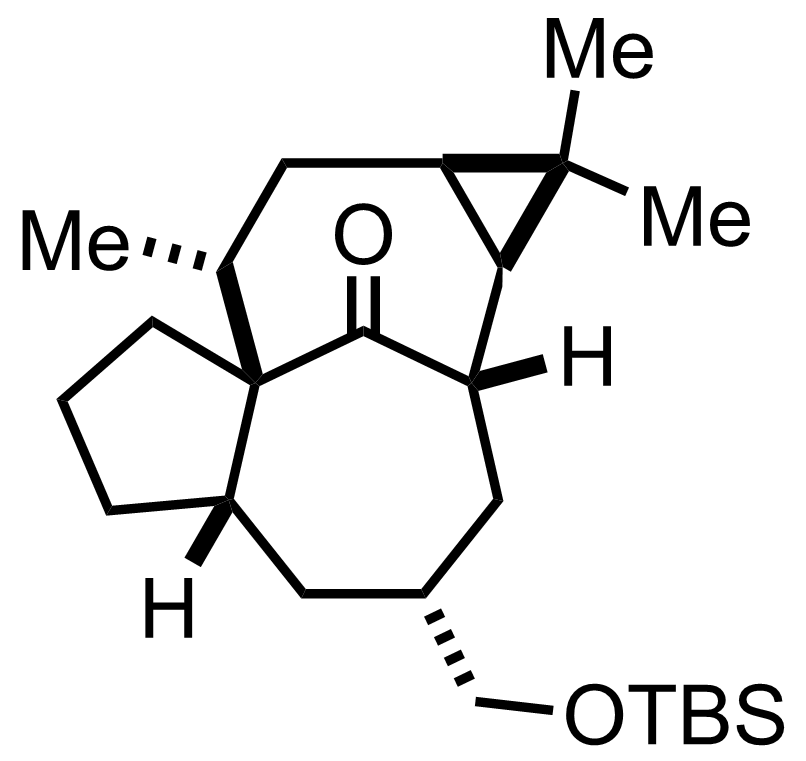
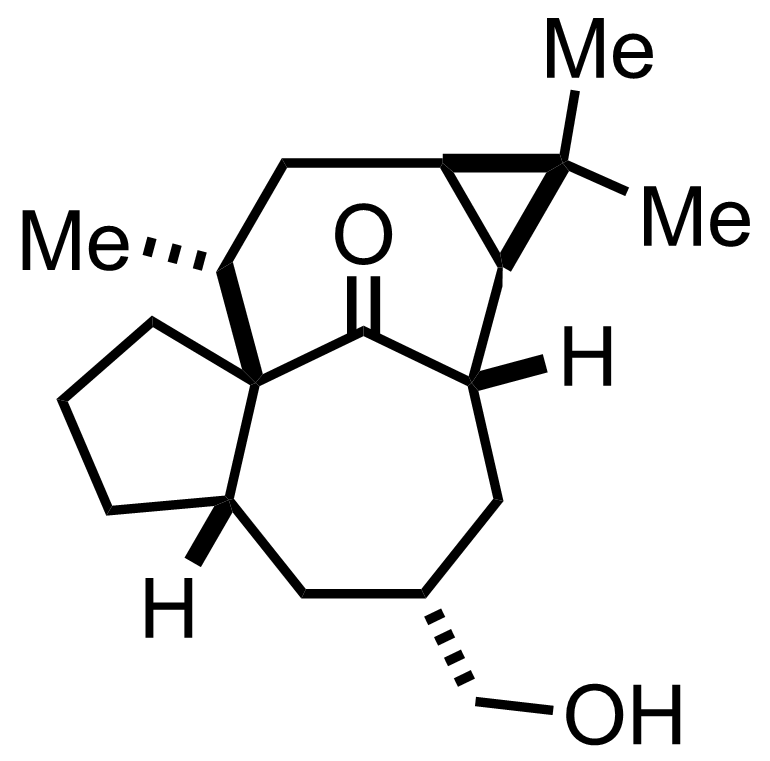


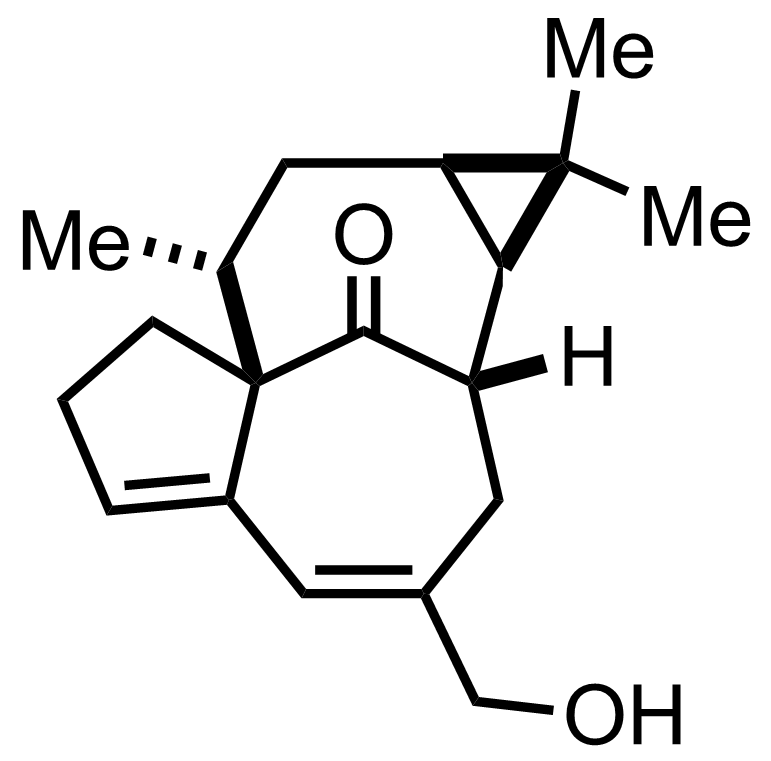
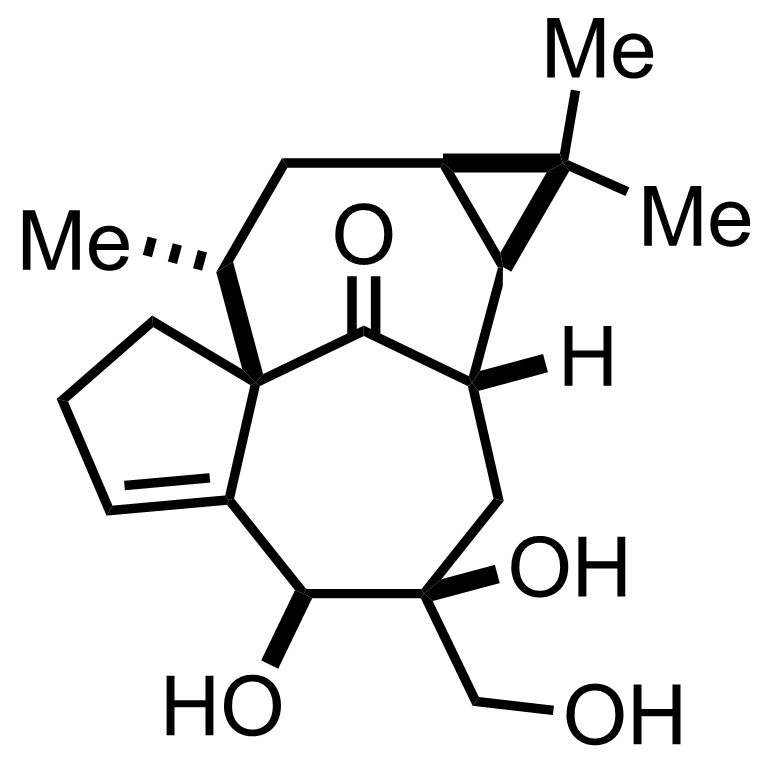
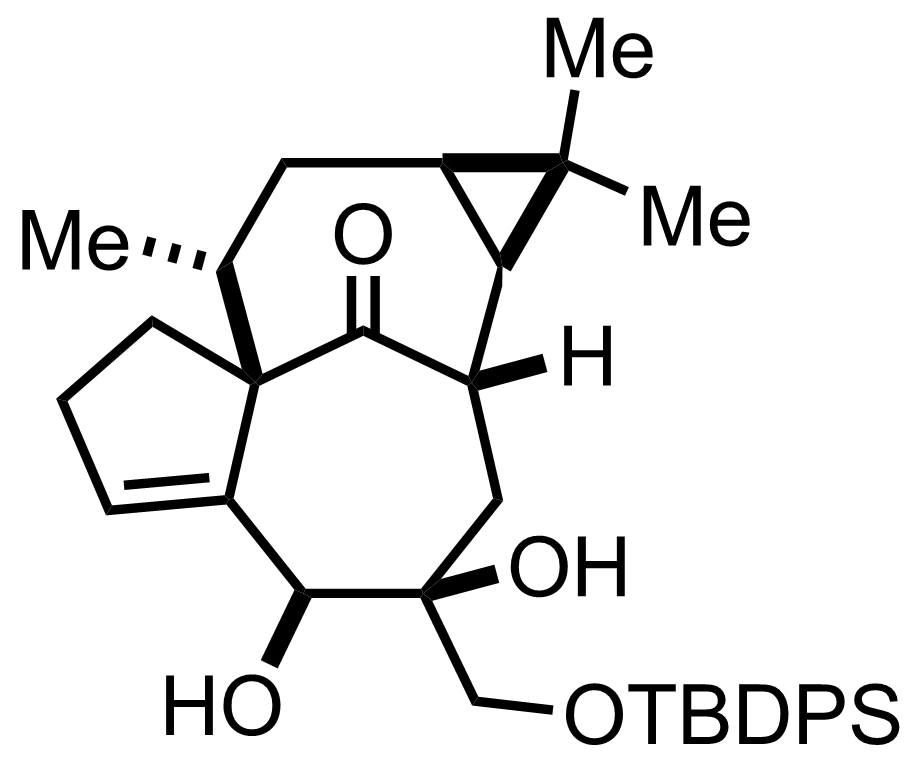
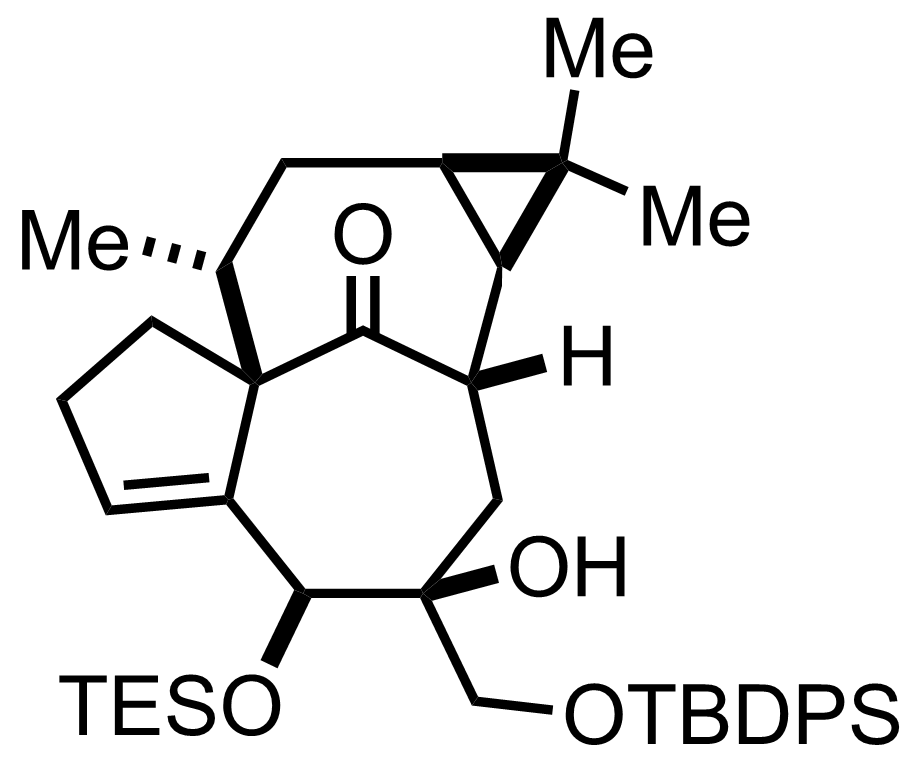


 +
+
 +
+

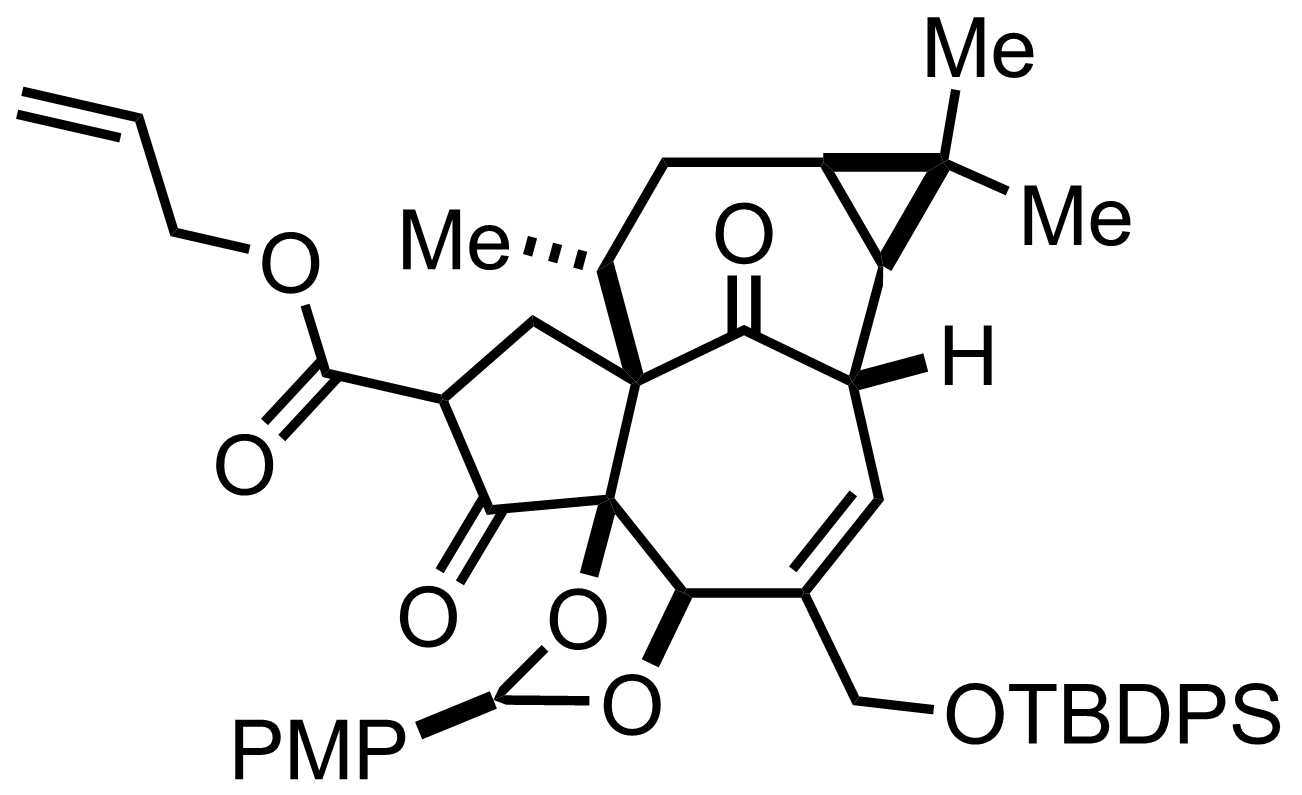
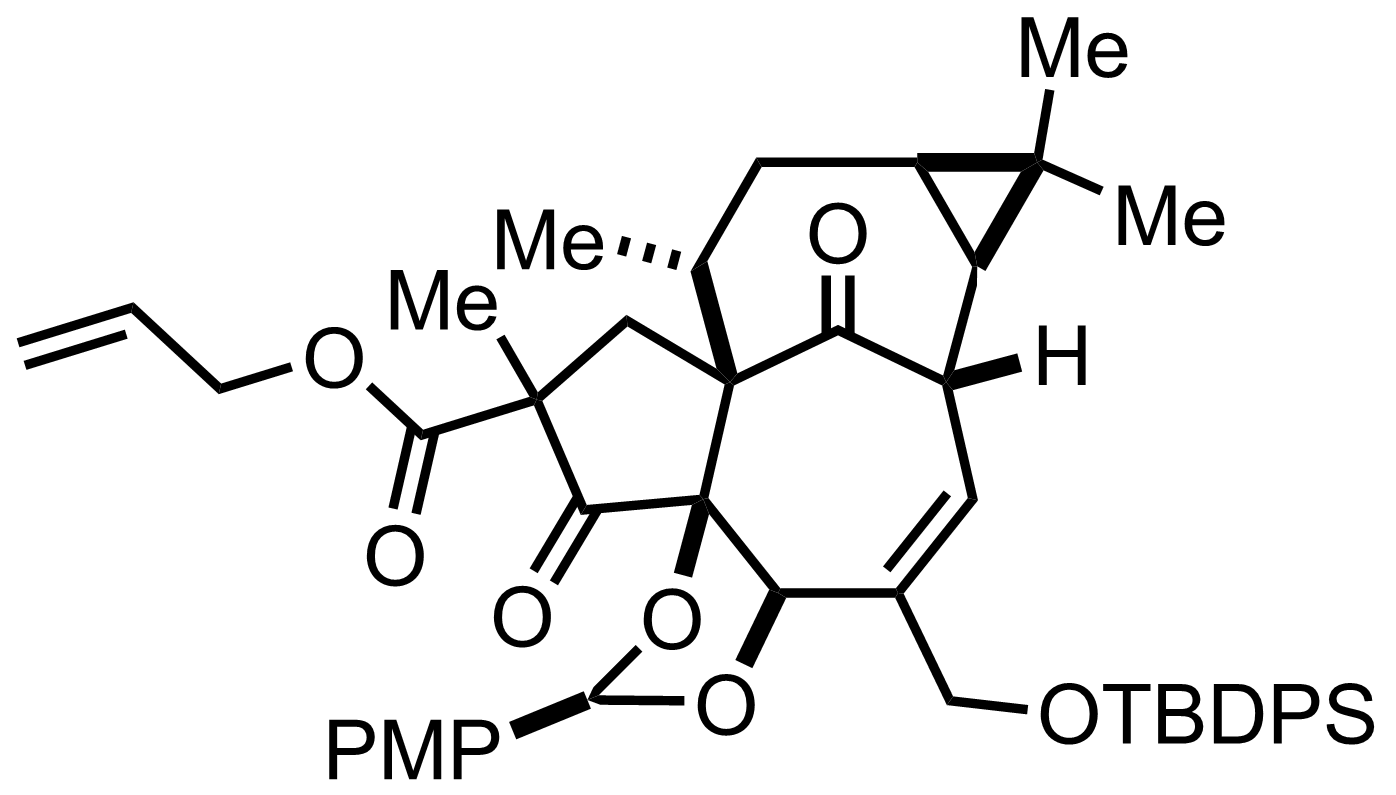

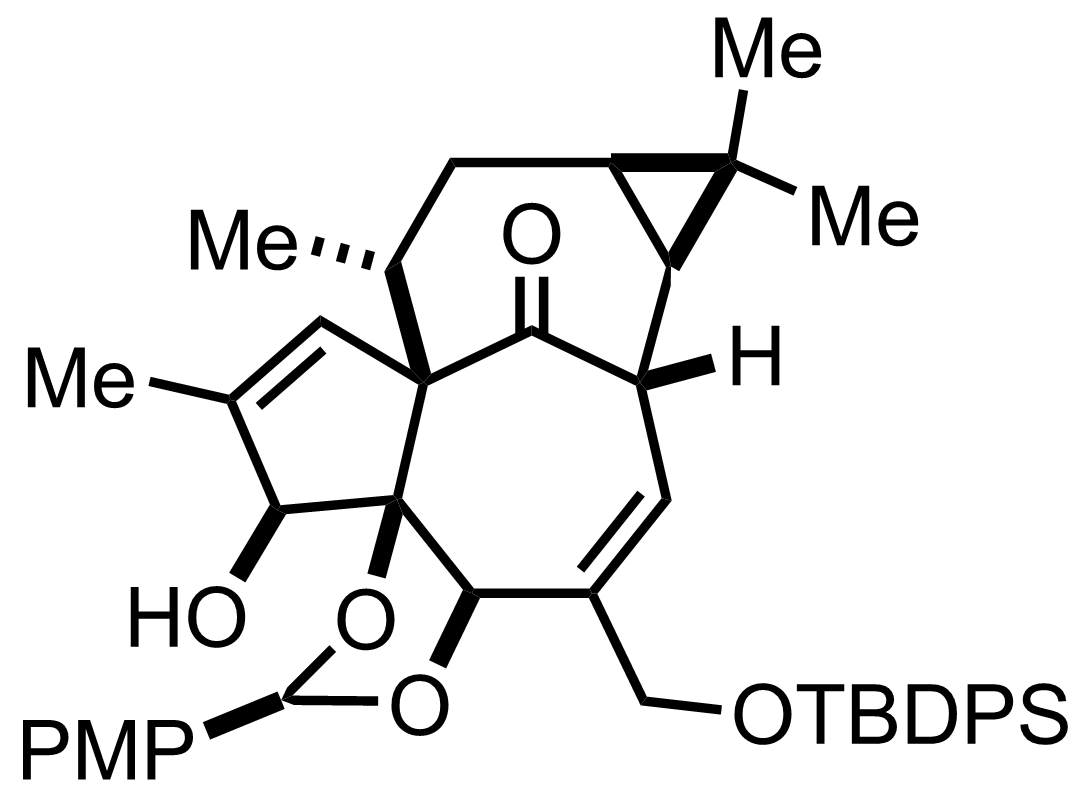


 +
+
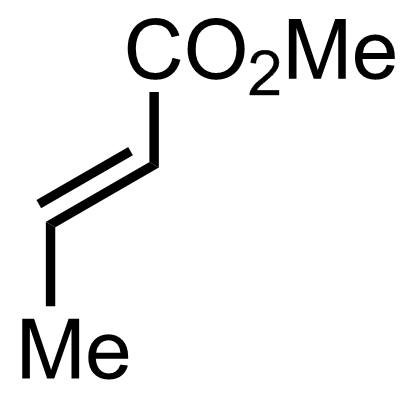

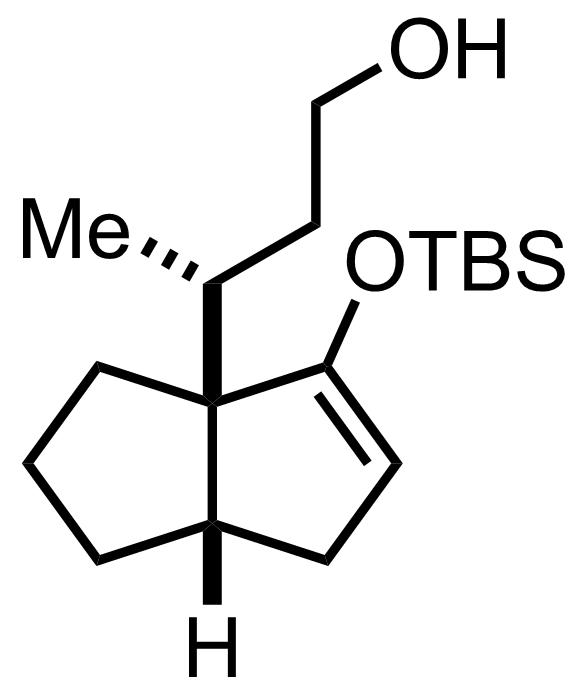
TBSOTf,
Et3N
CH2Cl2
RT, ON, 69% (2 steps)

LiAlH4
Et2O
0 °C to RT, 20 min, 100%

TsCl,
Et3N,
DMAP
CH2Cl2
0 °C to RT, ON, 88%

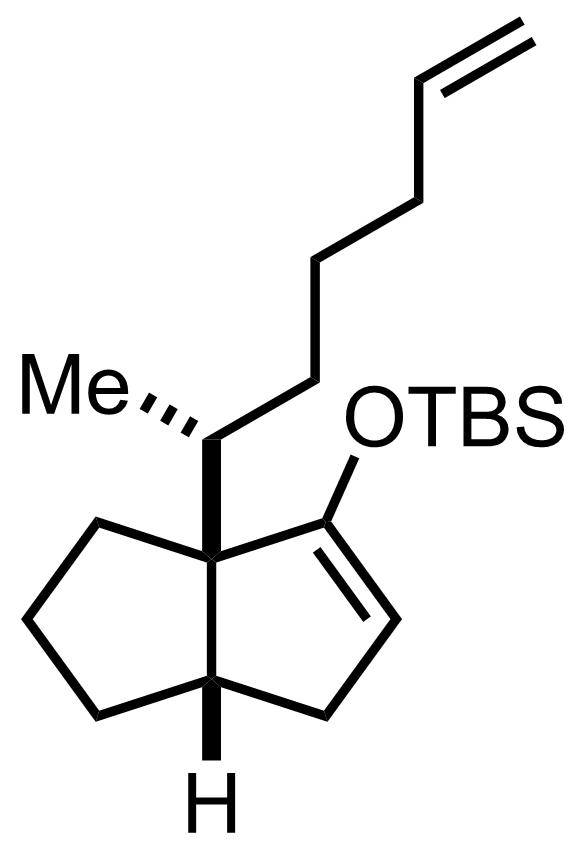
AllylMgBr,
CuI
Et2O
-20 to 0 °C, 90 min, 87%

HF
THF
RT, ON, 83%


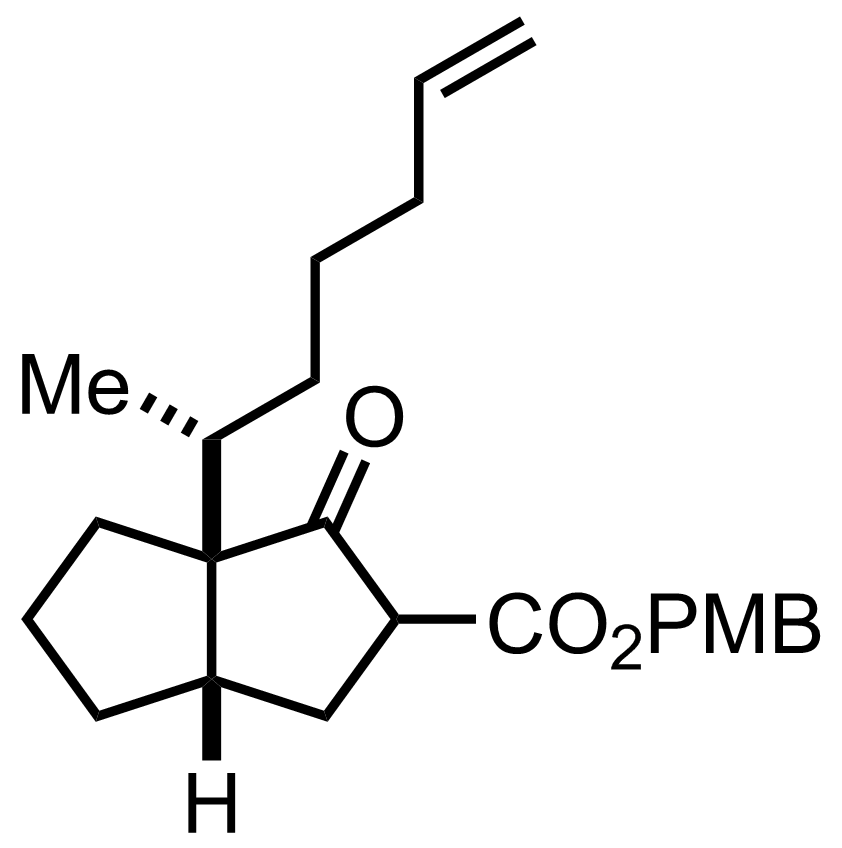
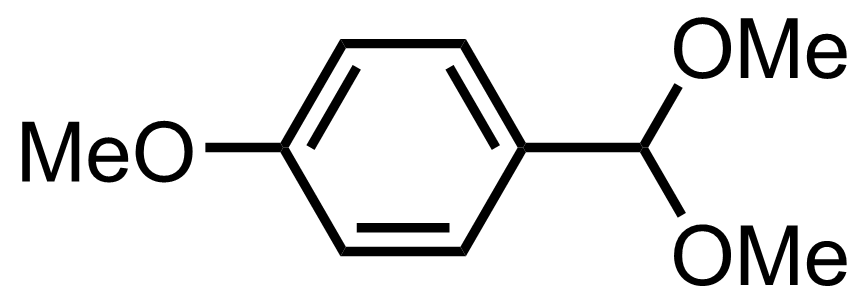
(4-MeOPh)CH2OH
Dean-Stark
PhMe
Reflux, 11 h, 97%

(CF3CO)2O,
CF3CO2H
Acetone
-78 °C to RT, ON, 93%

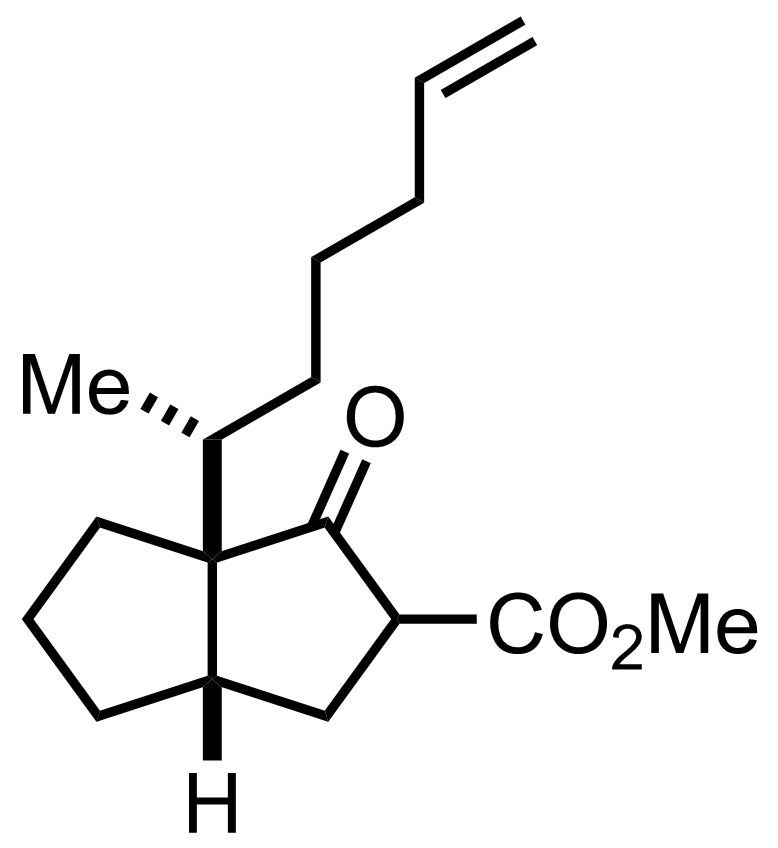
t-BuOOH,
SeO2,
Salicylic acid
CH2Cl2
Reflux, ON, 71%


 +
+

 +
+

LiAlH4
THF
RT, 40 min
 +
+

DBU
200 °C, 24 h

TBSCl,
Imidazole,
DMAP
CH2Cl2
RT, 14 h, 35% (4 steps)
"Also isolated was the C6-epimer (10% yield)."

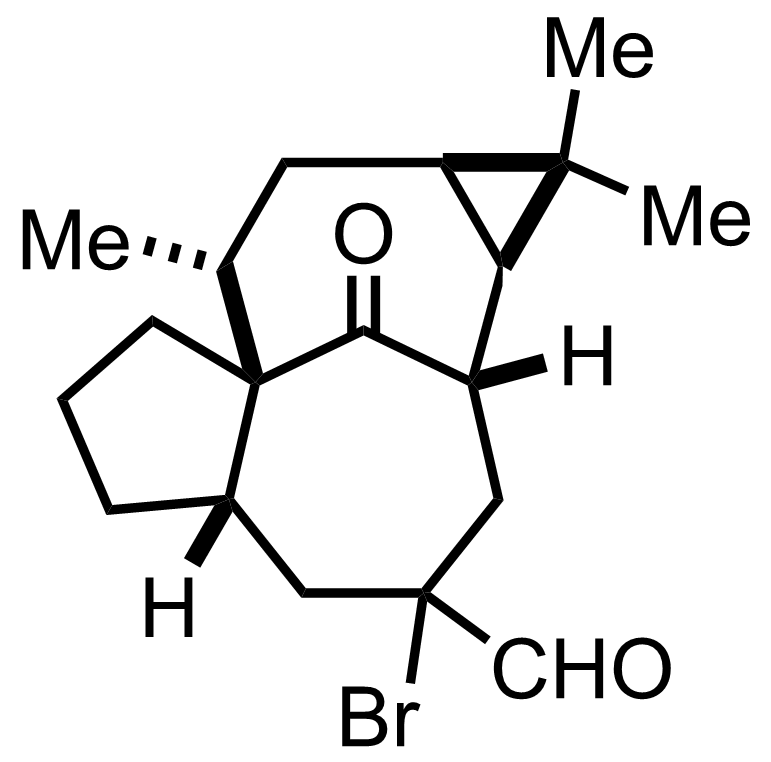
CHBr3,
Et3NBn+ Cl-,
NaOH
H2O
RT, 15 min, 100%


n-Bu4N+ F-
THF
RT, 3 h, 99%


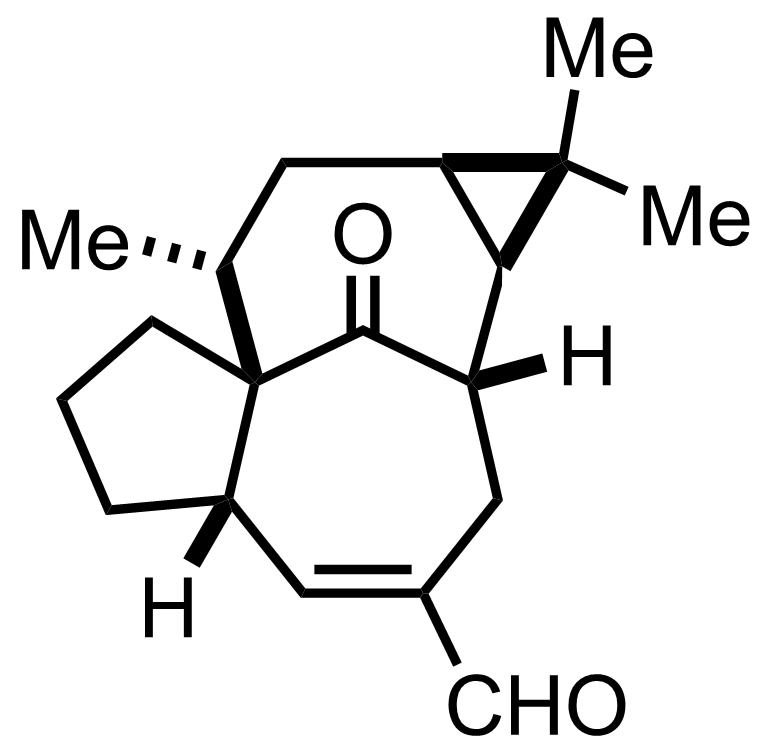
t-BuBr
DMSO
Reflux, 10 h

LiCl
DMF
Reflux, 60 min, 73% (2 steps)

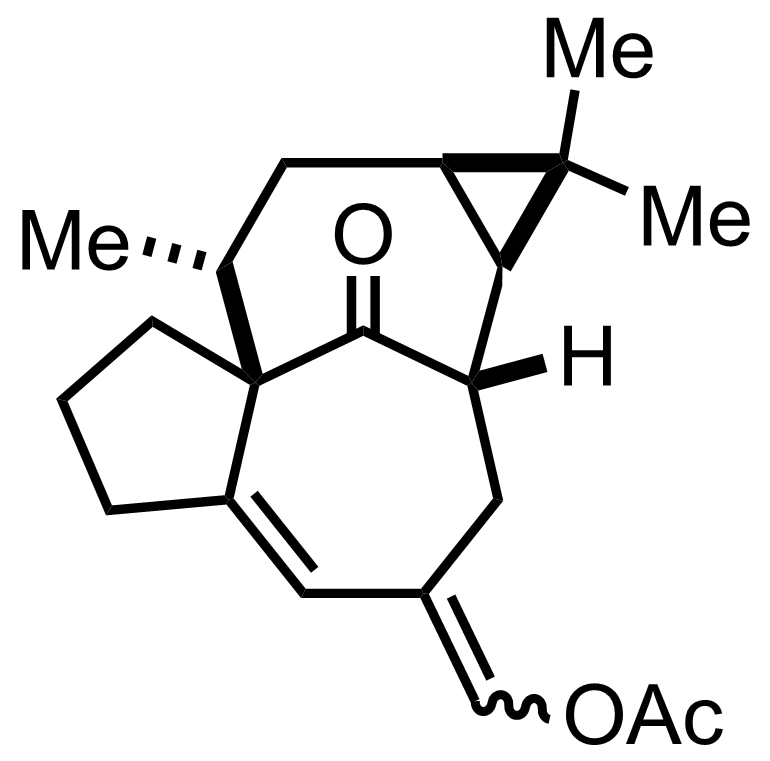
AcCl
Ac2O, Pyr
Reflux, 12 h, 80%

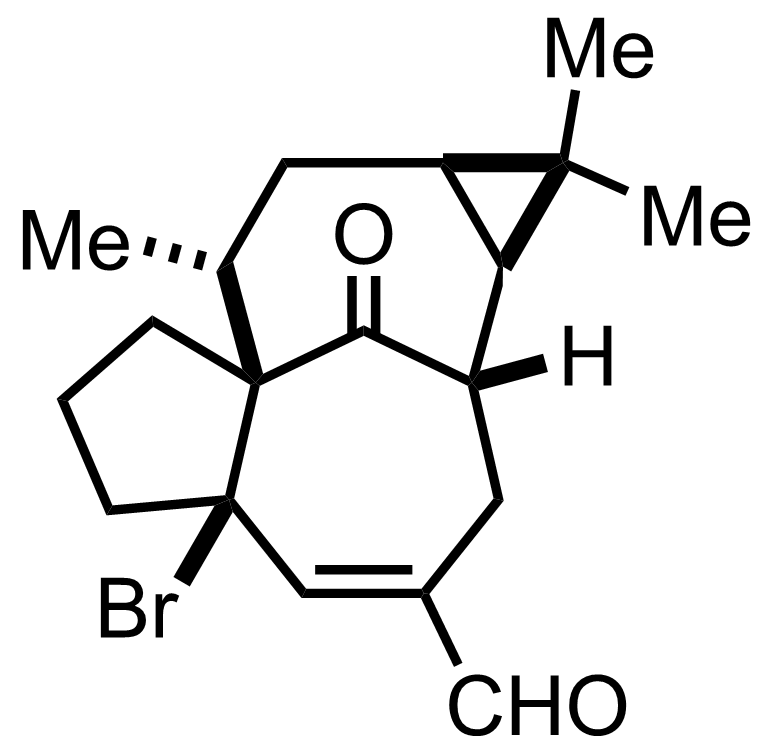
Pyr,
NBS,
NaOAc
Acetone
0 °C to RT, 12.5 h

LiCl
DMF
Reflux, 60 min, 50% (3 steps)

i-Bu2AlH
THF
-78 °C, 30 min, 80%


TBDPSCl,
Imidazole,
DMAP
CH2Cl2
RT, 12 h, 85%

TESOTf,
2,6-Lutidine
CH2Cl2
RT, 30 min, 83%

OsO4
Pyr
0 °C to RT, 12 h, 93%

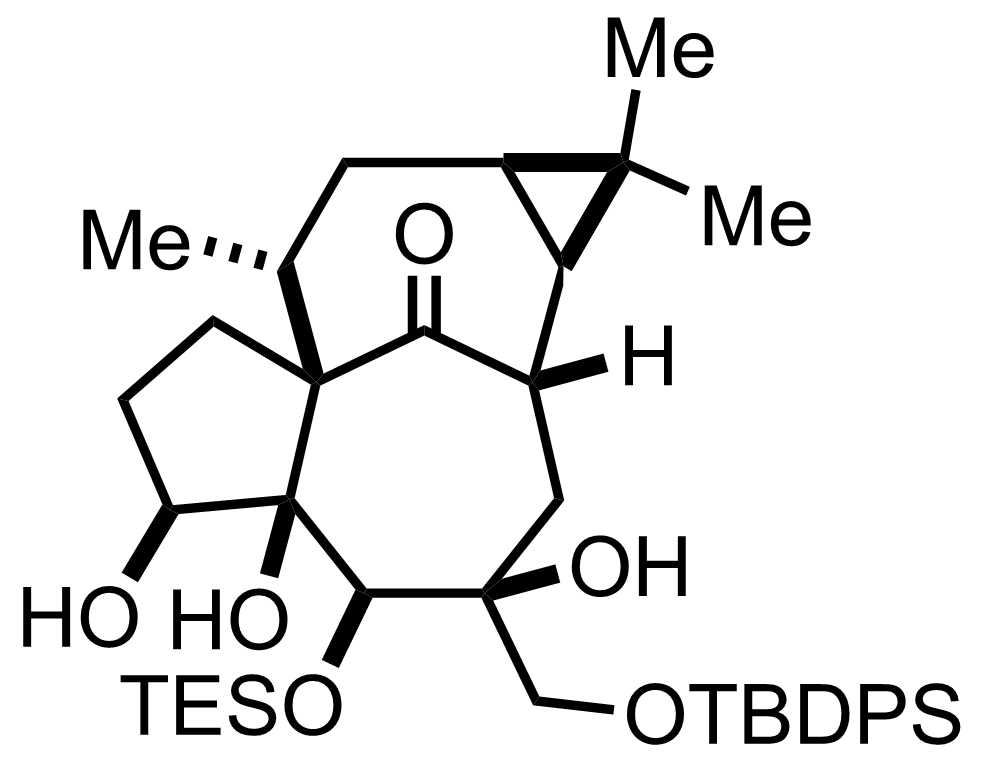
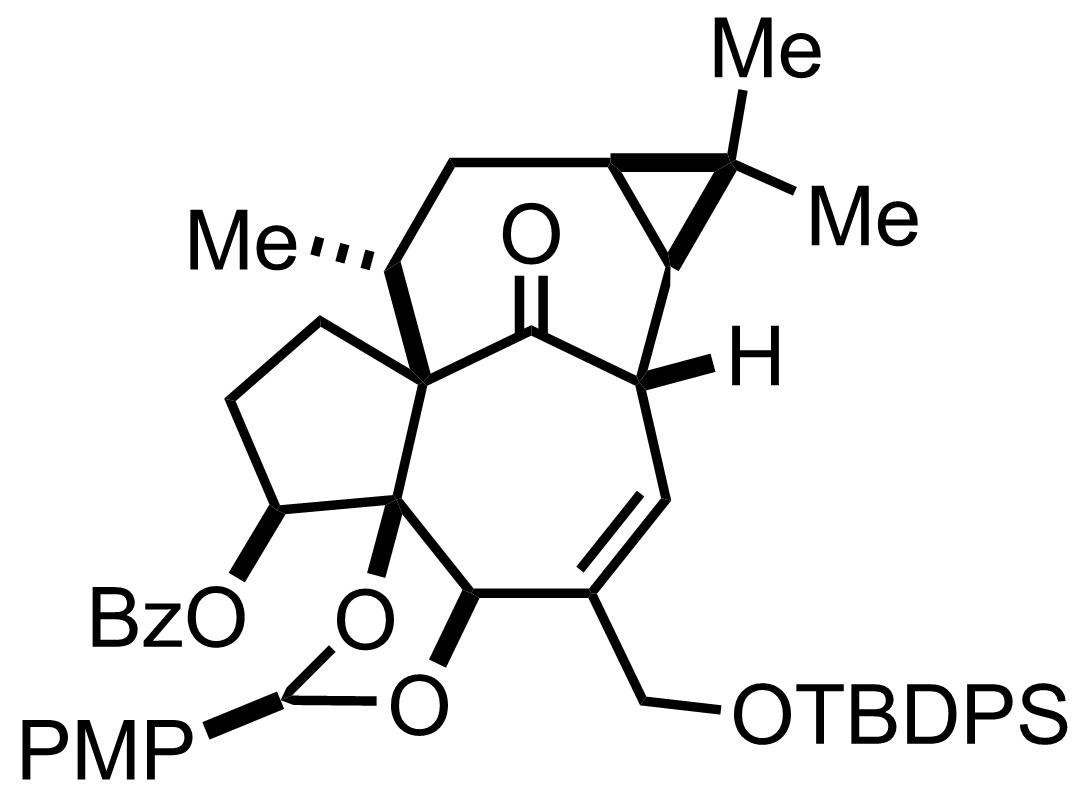
DMAP,
BzCl,
Et3N
CH2Cl2
0 °C to RT, 30 min, 82%

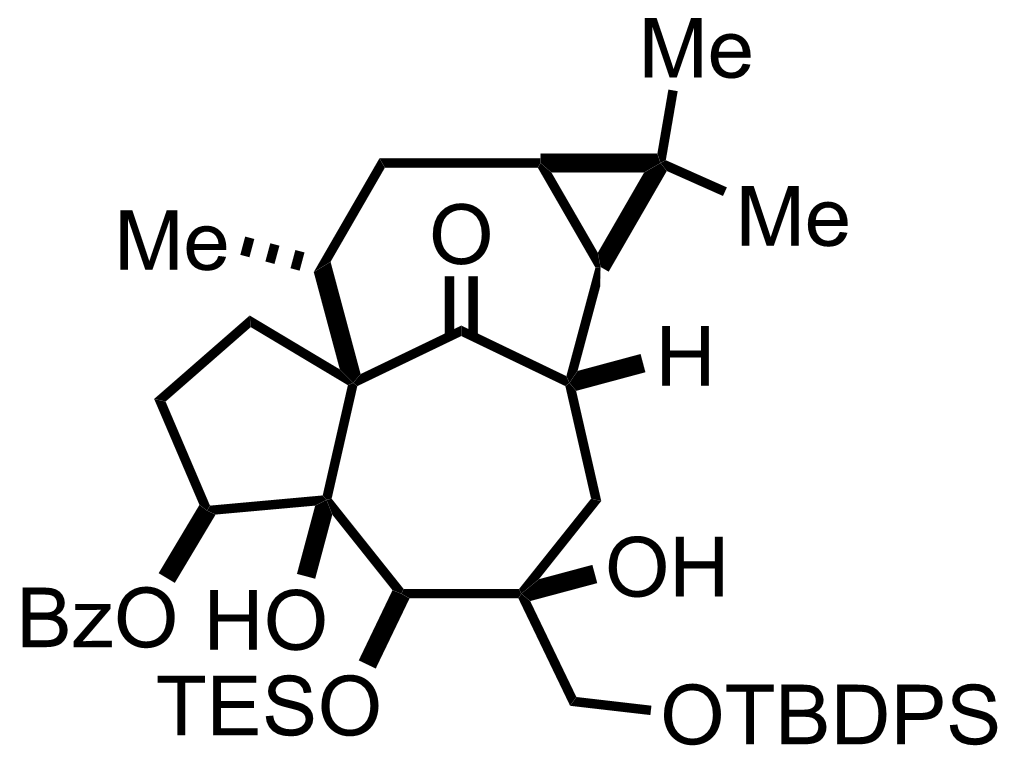
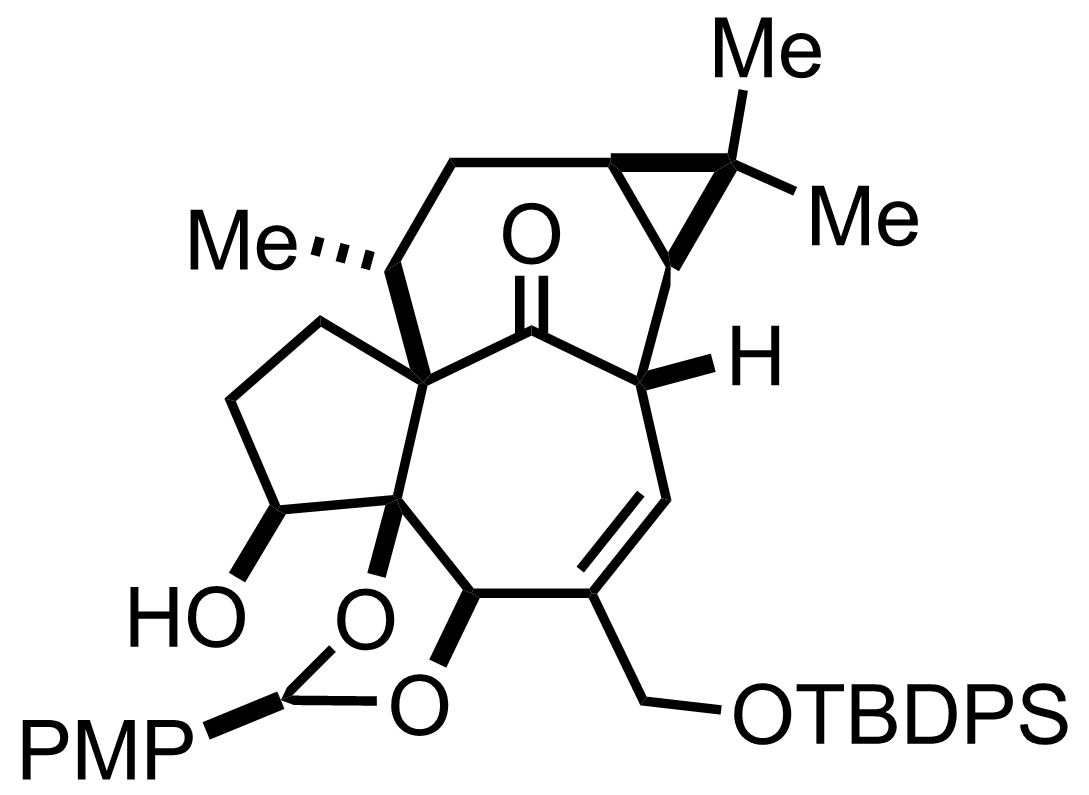
Pyr,
SOCl2
CH2Cl2
RT, 10 min

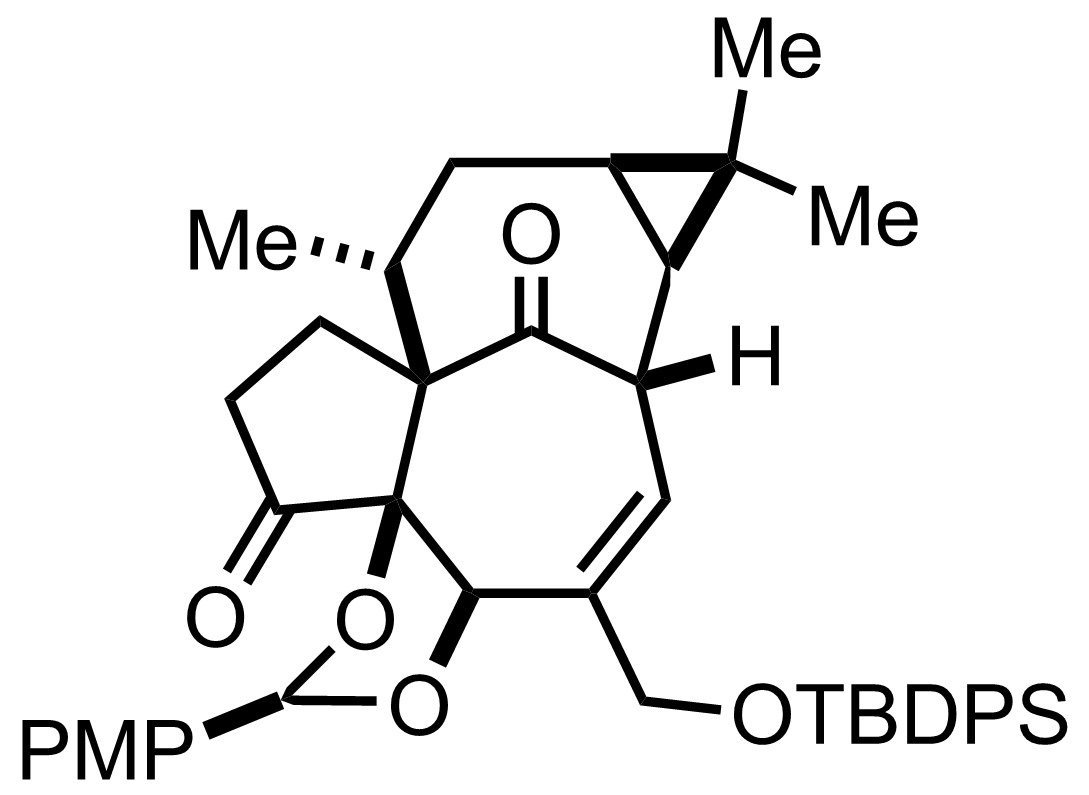
RuCl3,
NaIO4
CCl4, H2O, MeCN
RT, 10 min, 79% (2 steps)

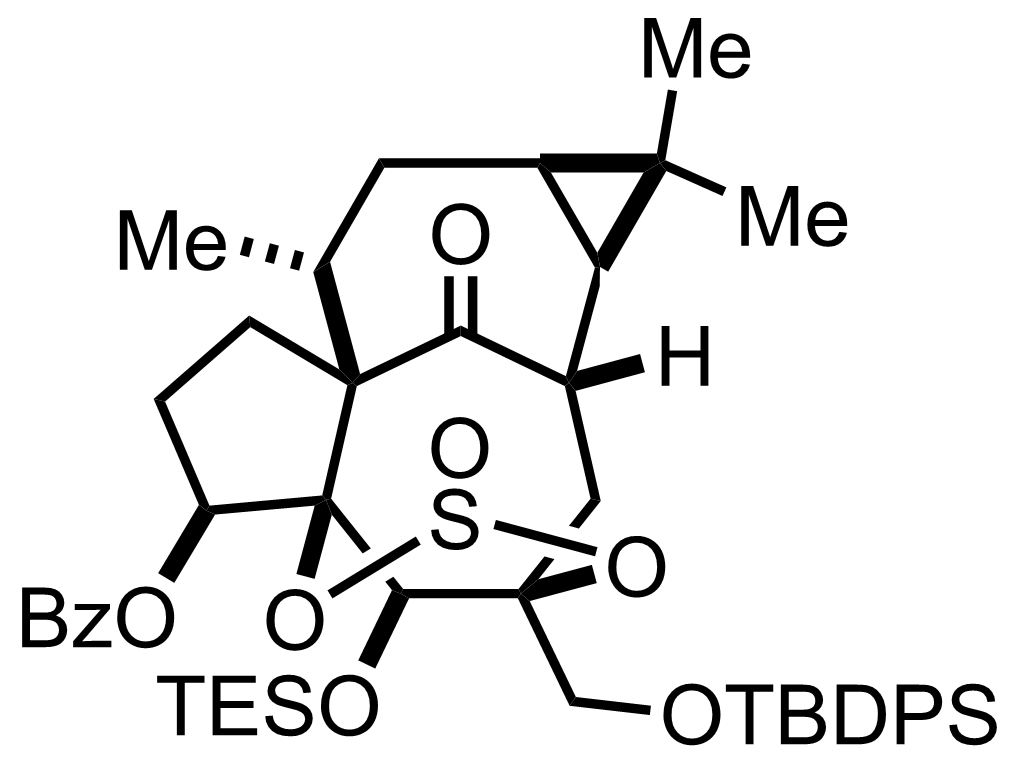
DBU
PhMe
Reflux, 12 h

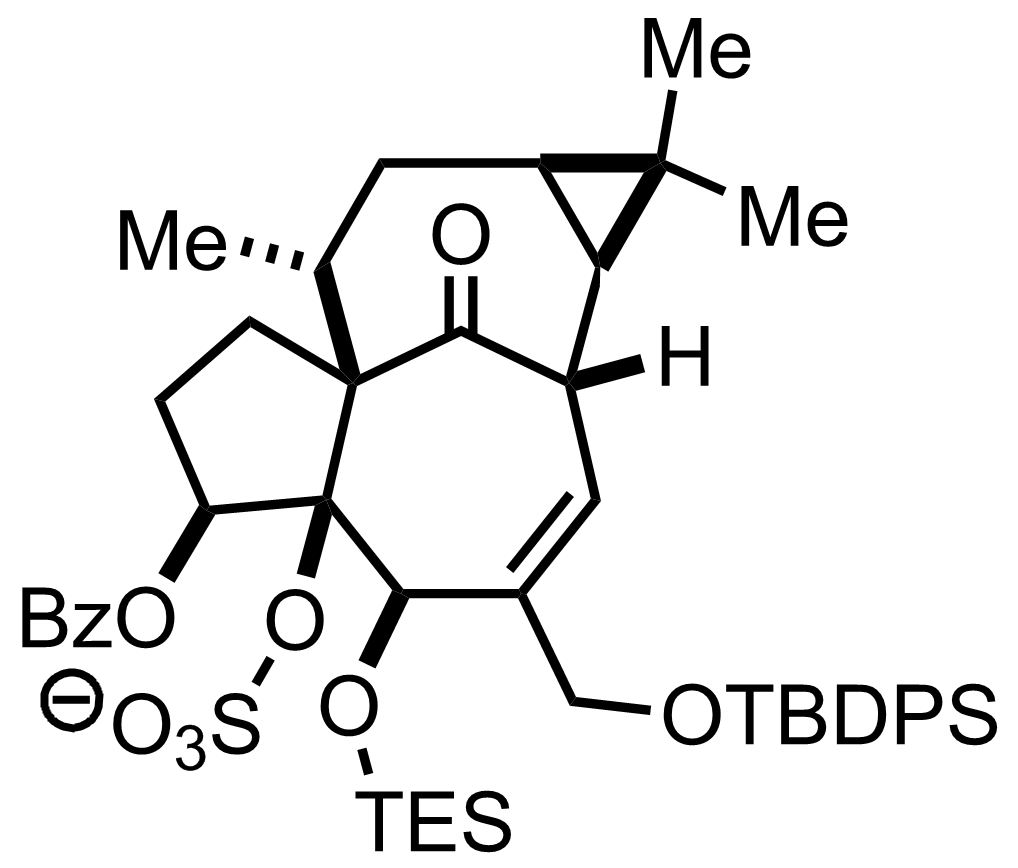
H2SO4
H2O, MeCN, THF
RT, 60 min, 34% (2 steps)
"Also isolated was the desired TES-deprotected alcohol (40 % yield, see next step)"

n-Bu4N+ F-
THF
-78 °C, 4 h, 72%
 +
+

Camphorsulfonic acid
CH2Cl2
RT, 30 min, 86%

K2CO3
MeOH, THF
RT, 2 h, 81%

 +
+


MeI,
K2CO3
Acetone
RT, 2 h

Pd(OAc)2
MeCN
80 °C, 2 h, 20% (3 steps)
"The yield was 23 % brsm."


HCl
MeOH
RT, 5 h

n-Bu4N+ F-
THF
RT, 15 min, 28% (3 steps)

