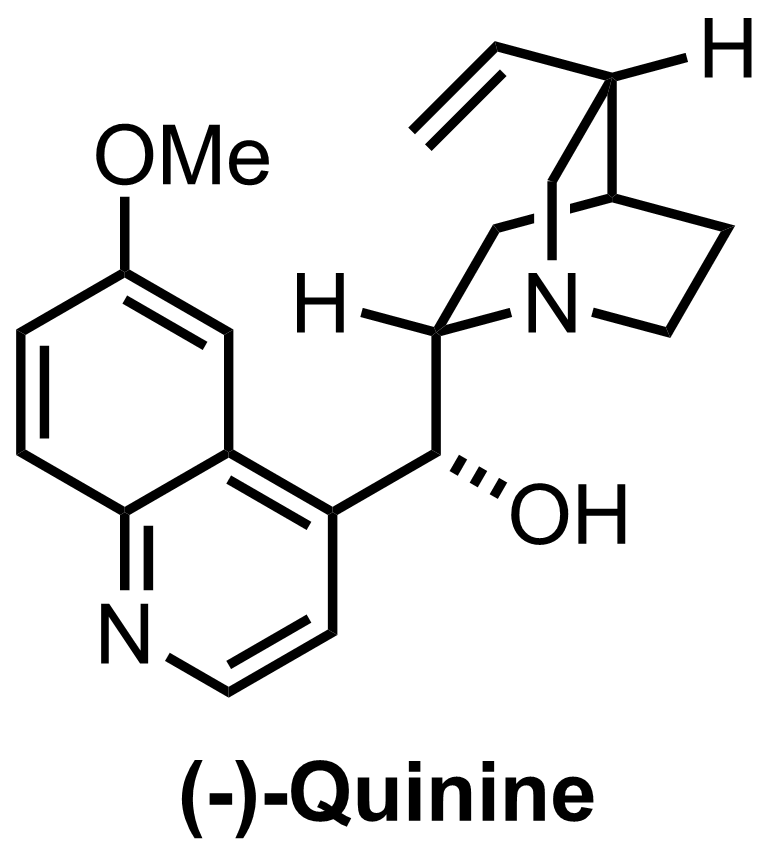
Synthesis of Quinine
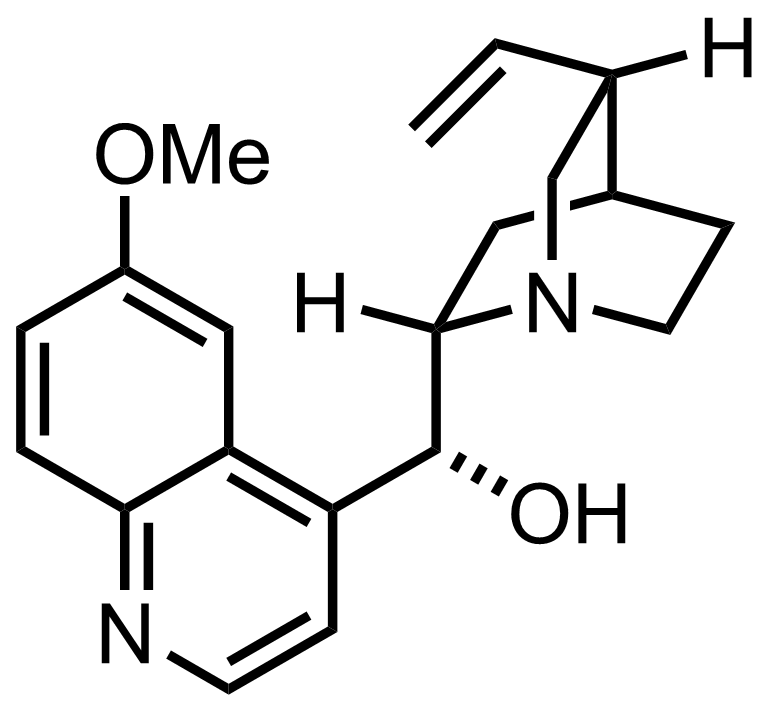
C20H24N2O2
| Principal investigator | Milan Uskokovic |
|---|---|
| Publication year | 1978 |
| Synthesis type | Total |
| Number of steps | 14 (linear) |
| References |
Part 1 of 1
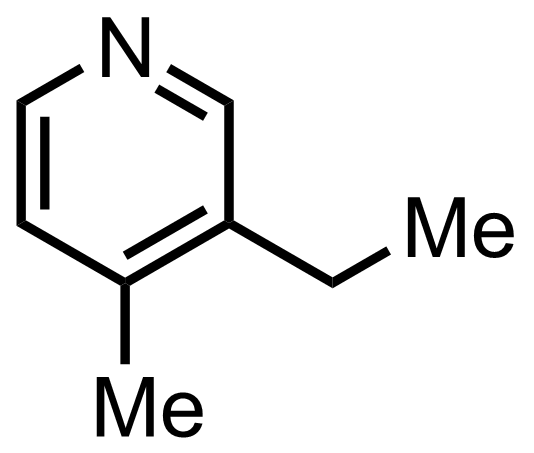
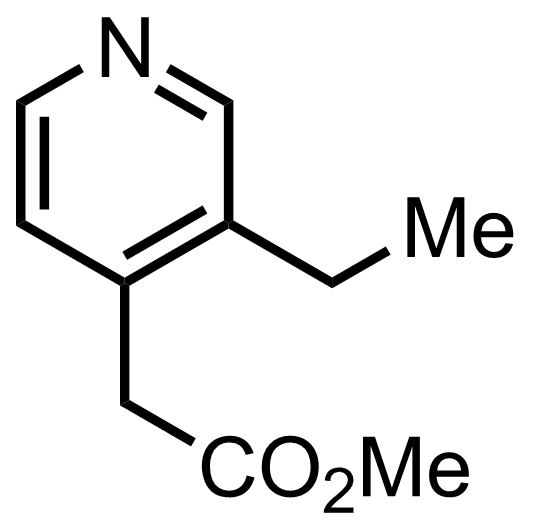
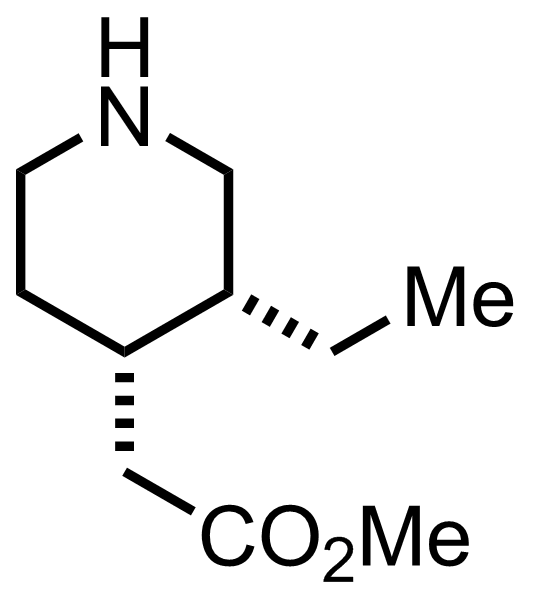
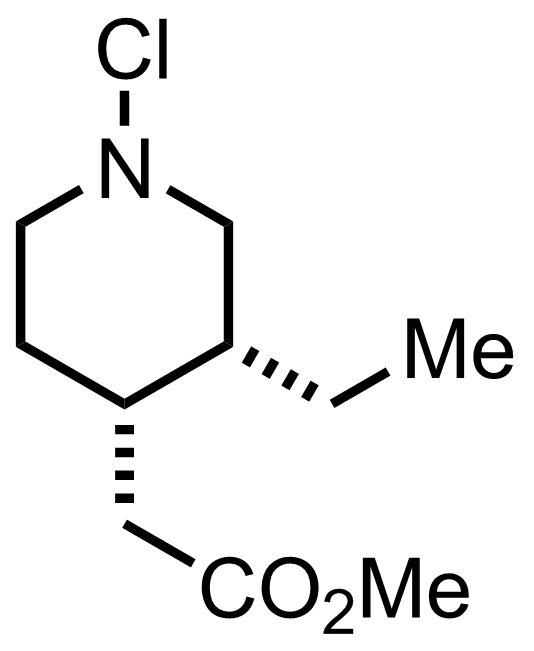
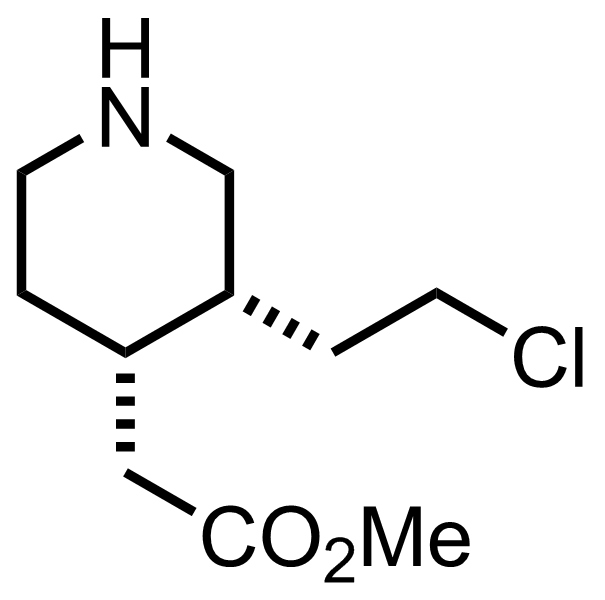
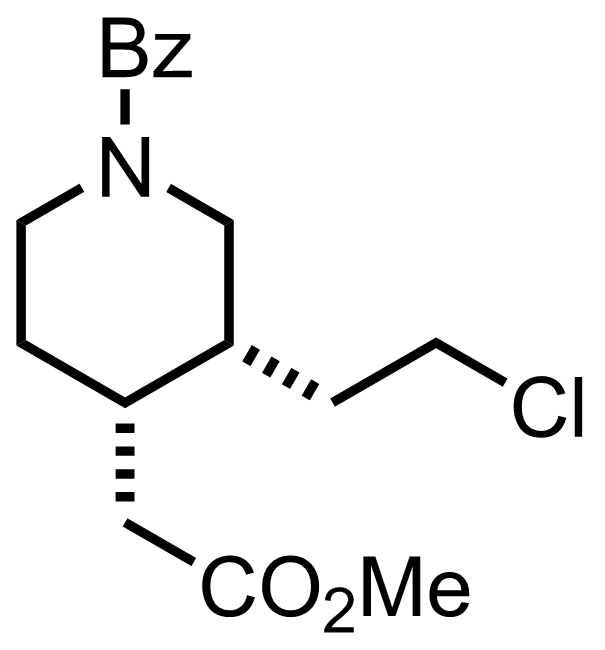
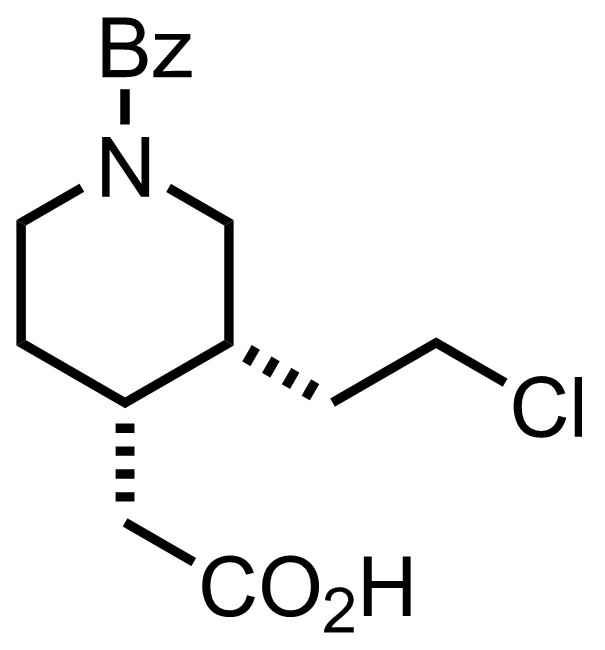
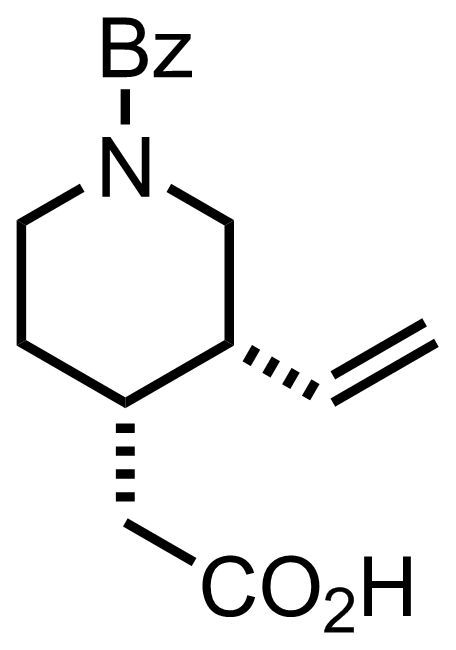
 +
+
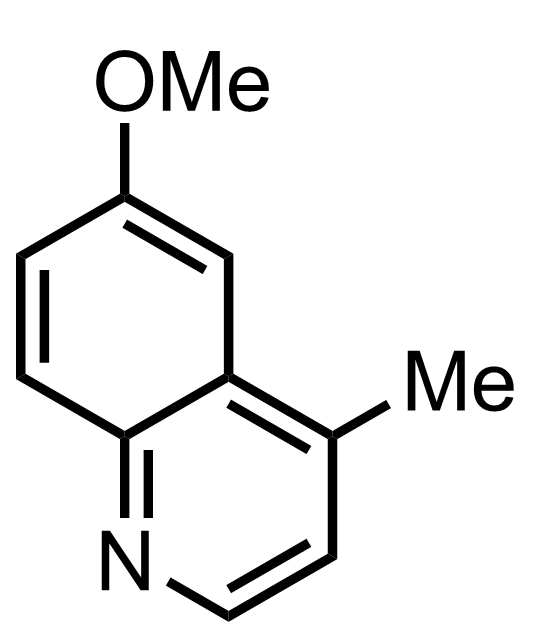
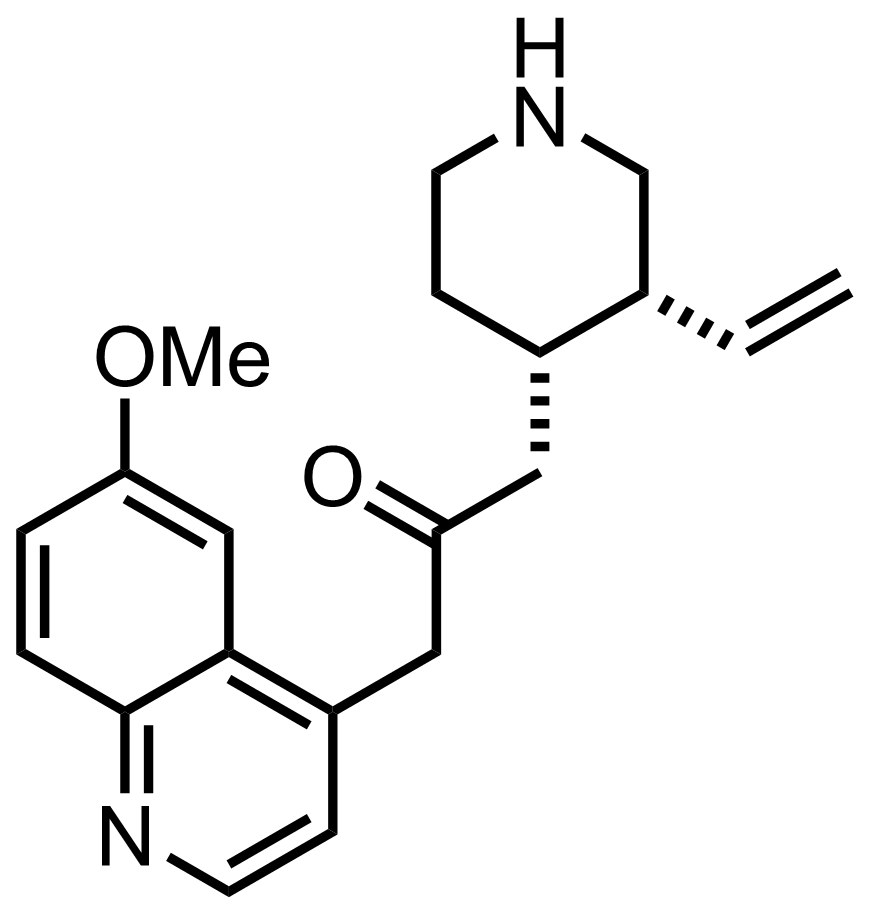
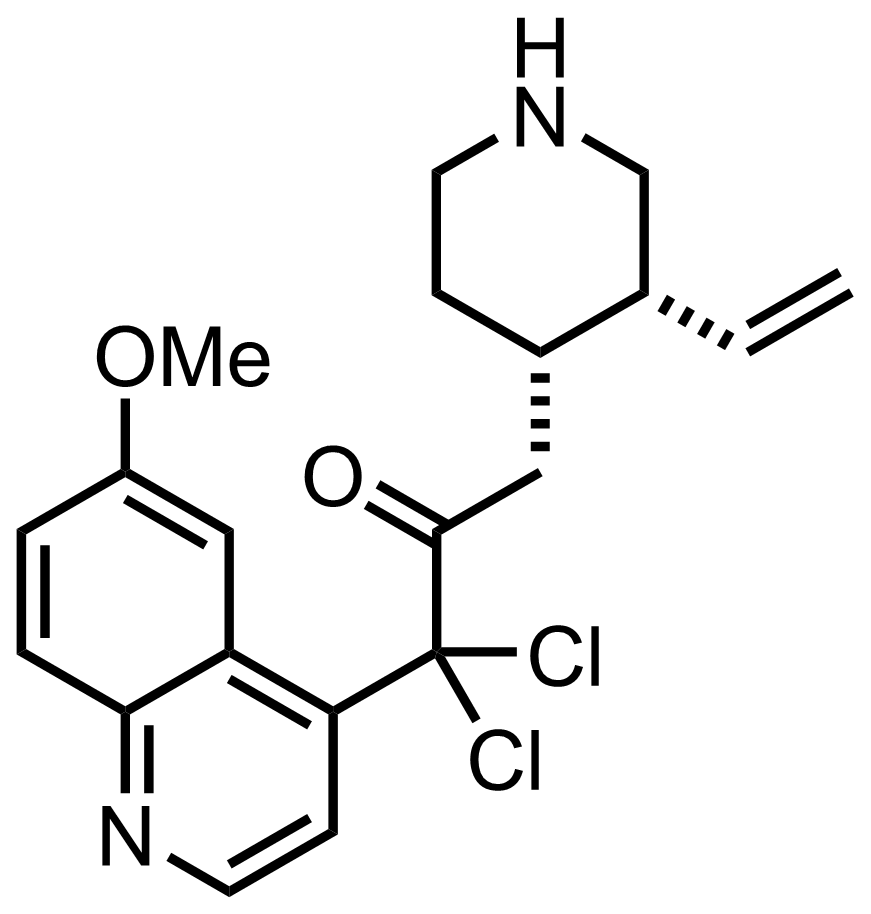
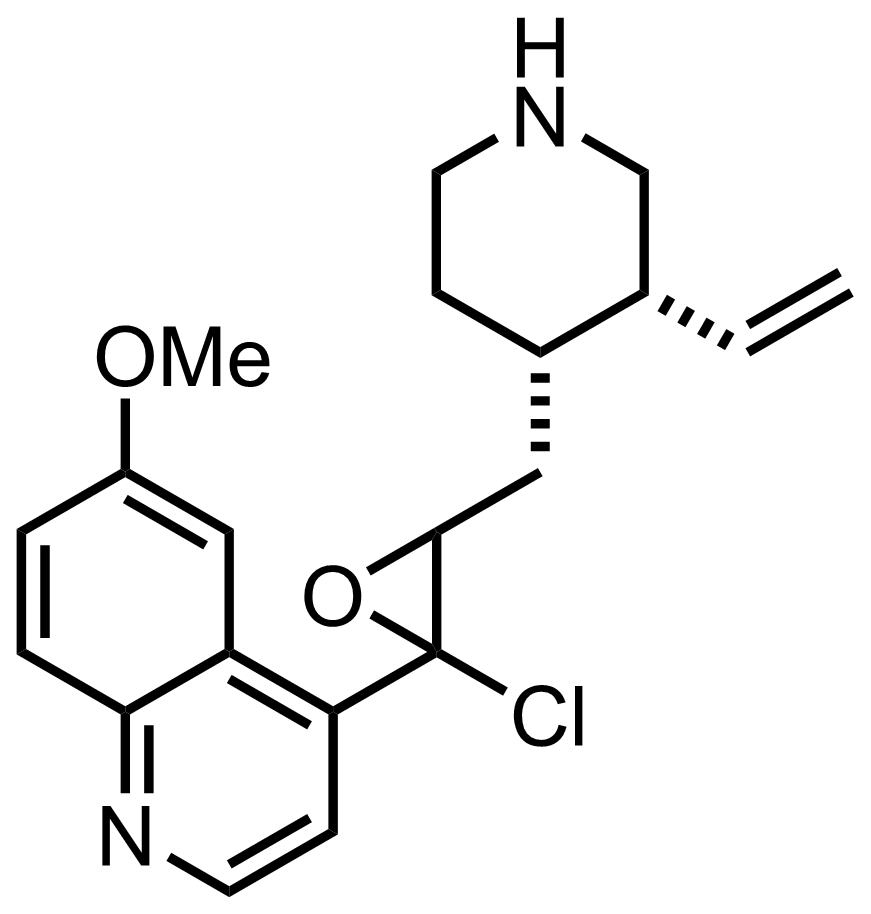
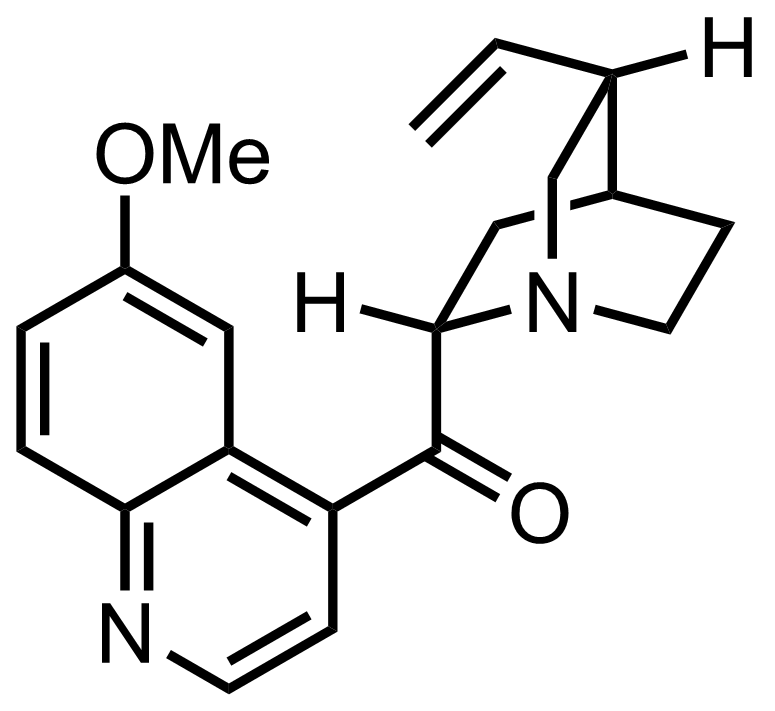

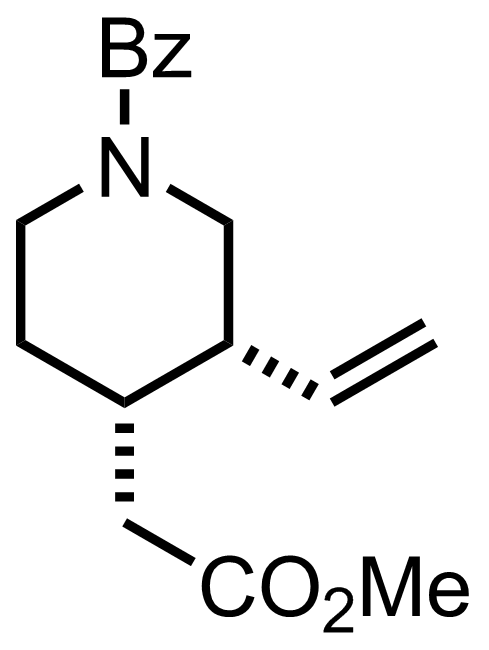
- i-Pr2NLi
- MeOCO2Me
THF
-78 °C to RT, 4 h, 99%

PtO2,
HCl,
H2
70 atm
H2O
60 °C, 82%
"The enantiomers were resolved with (-)-tartaric acid (29 % yield)."

NCS
Et2O
RT, 60 min, 92%



NaOH
H2O, MeOH
RT, 17 h, 99%

KOt-Bu
DMSO, PhH
70 °C, 7 h, 88%

CH2N2
Et2O, MeOH
RT, 90 min, 92%
 +
+

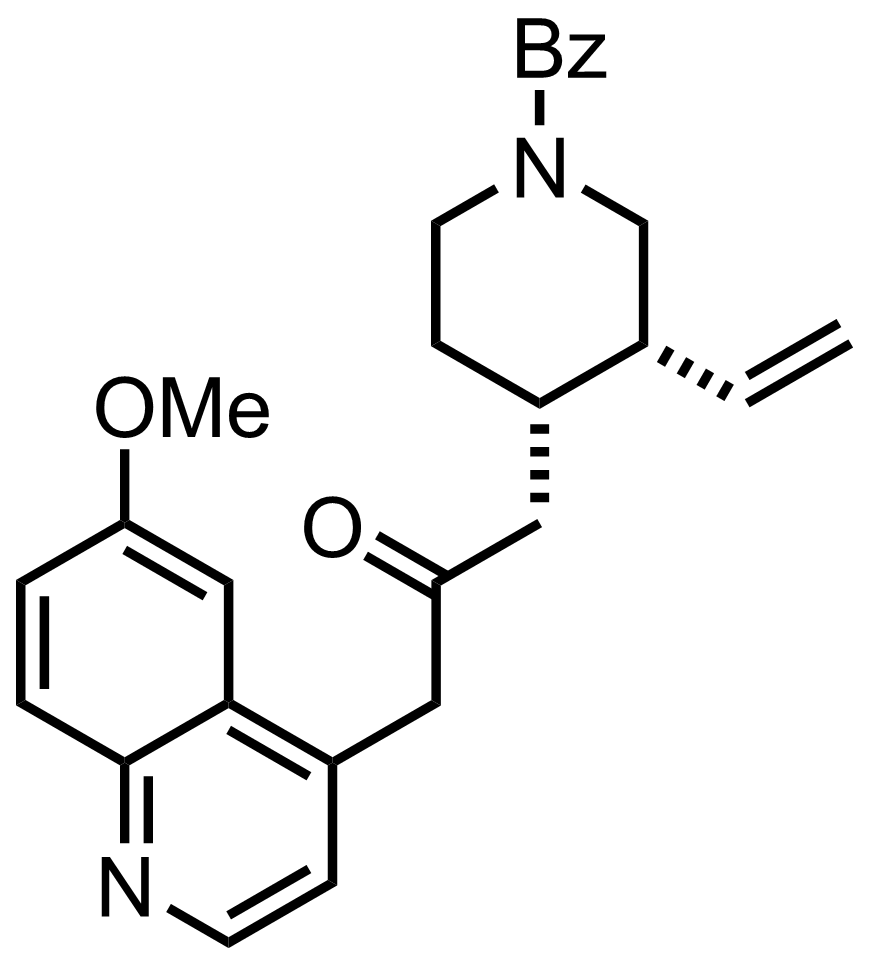
i-Pr2NLi
THF
-78 °C to RT, 60 min, 78%

H2SO4
H2O
Reflux, 24 h, 75%

i-Pr2NCl,
H3PO4
RT, 24 h

NaBH4
MeOH, THF
RT, 20 min

Ba(OH)2
H2O, MeOH
RT, 18 h, 27% (3 steps)

i-Bu2AlH
PhMe
-78 °C, 33%
"Quinine was selectively cristallized (as a tartrate salt) from the resulting two diastereoisomers."

