Synthesis of Quinine
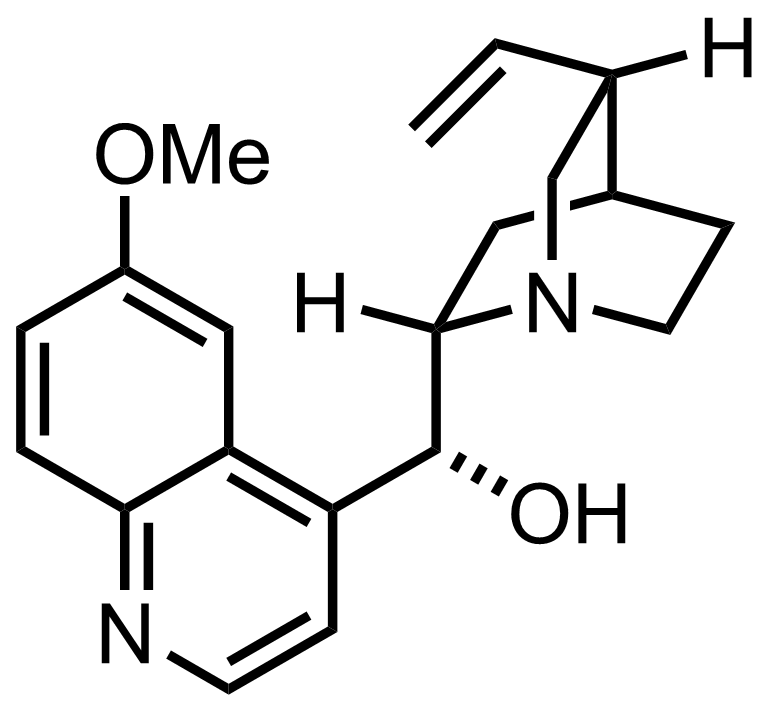
C20H24N2O2
| Principal investigator | Milan Uskokovic |
|---|---|
| Publication year | 1970 |
| Synthesis type | Total |
| Number of steps | 10 (linear) |
| References |
Part 1 of 1
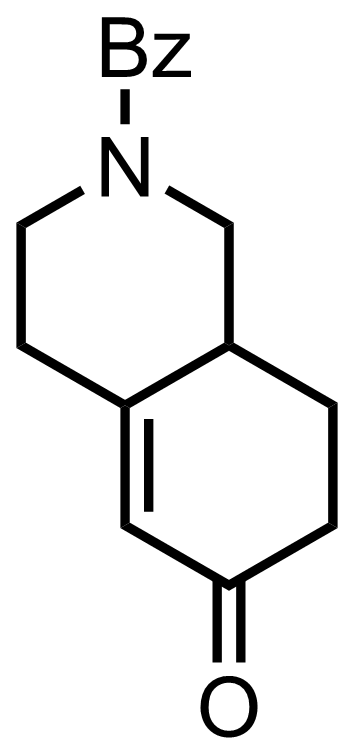
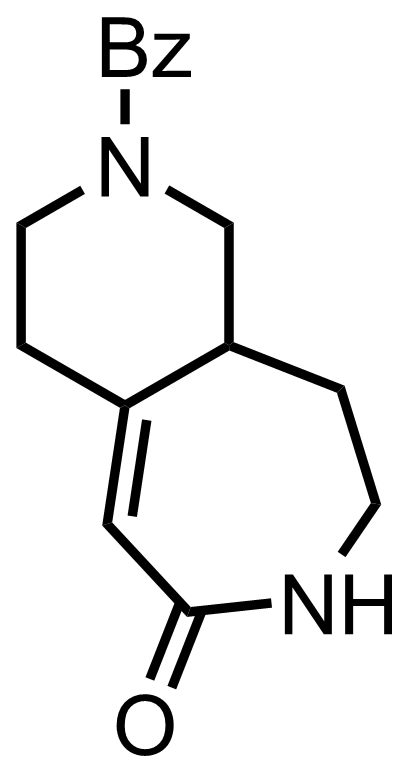


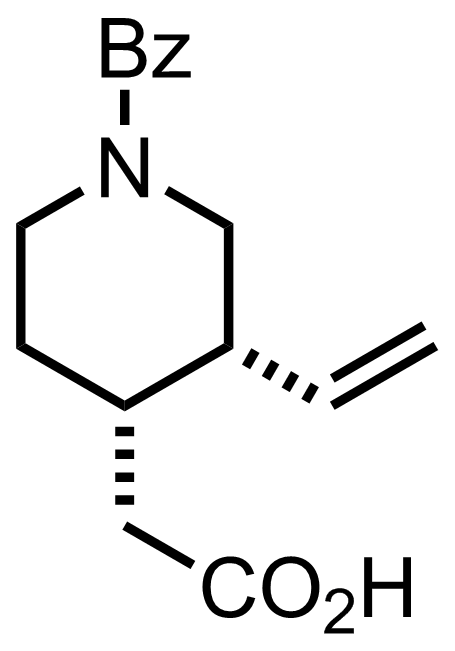
 +
+


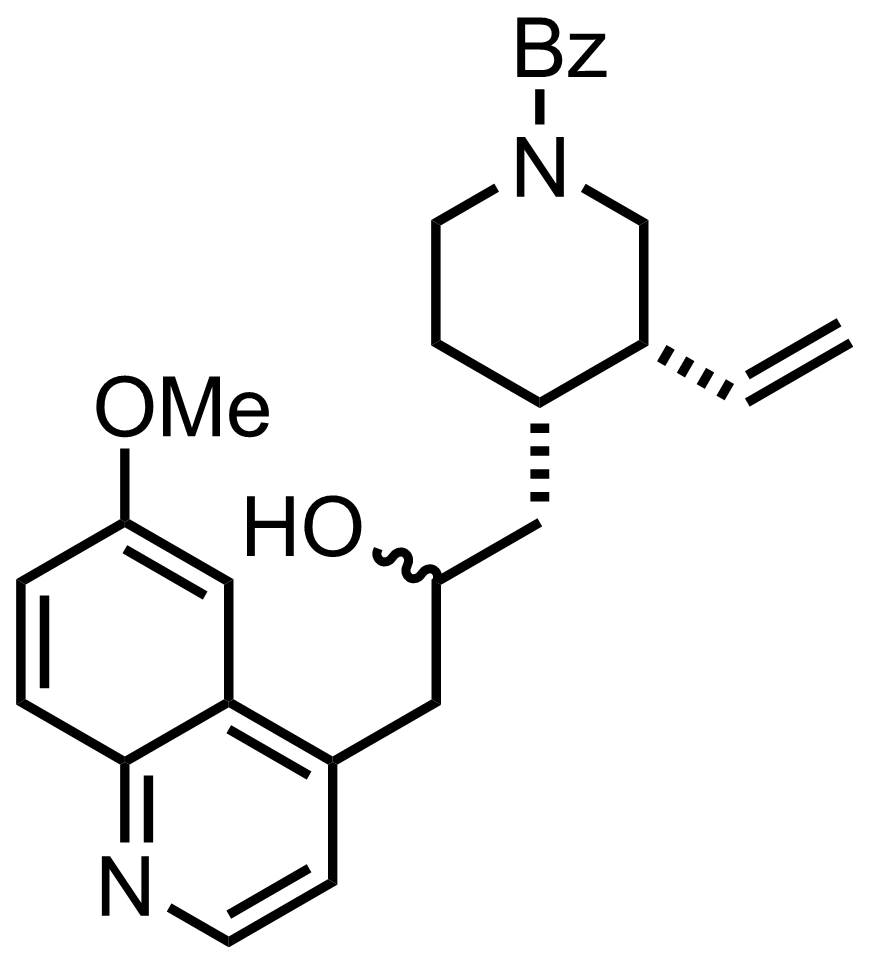
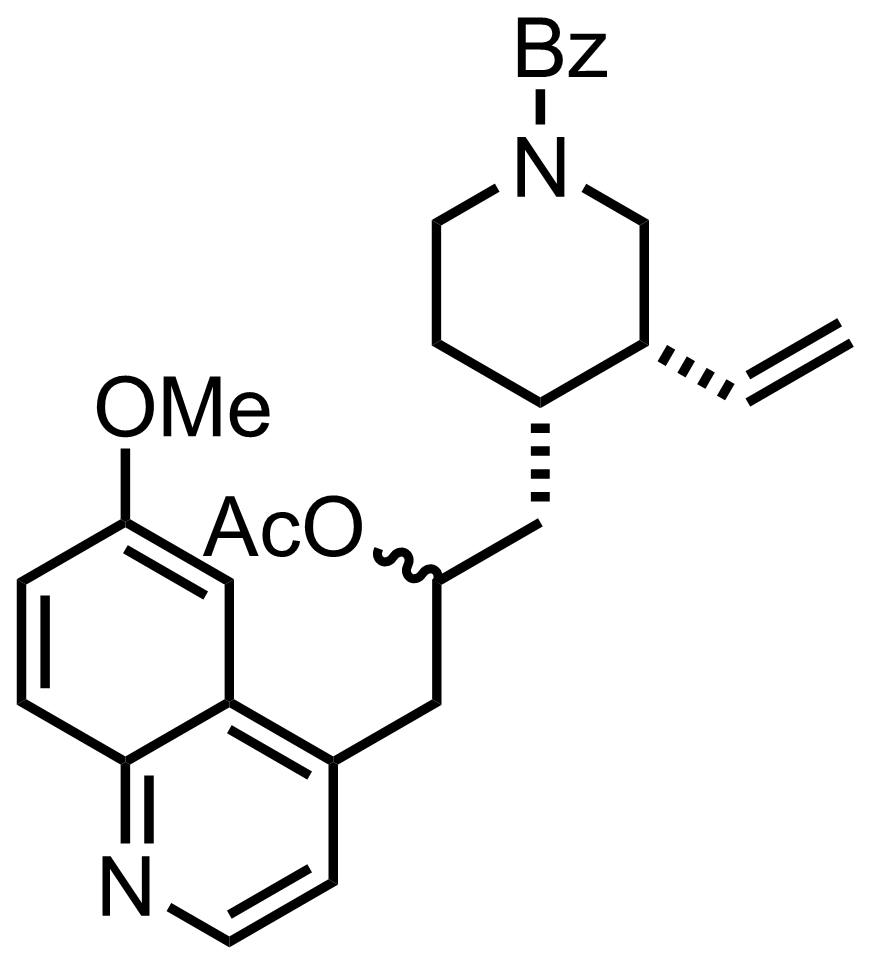
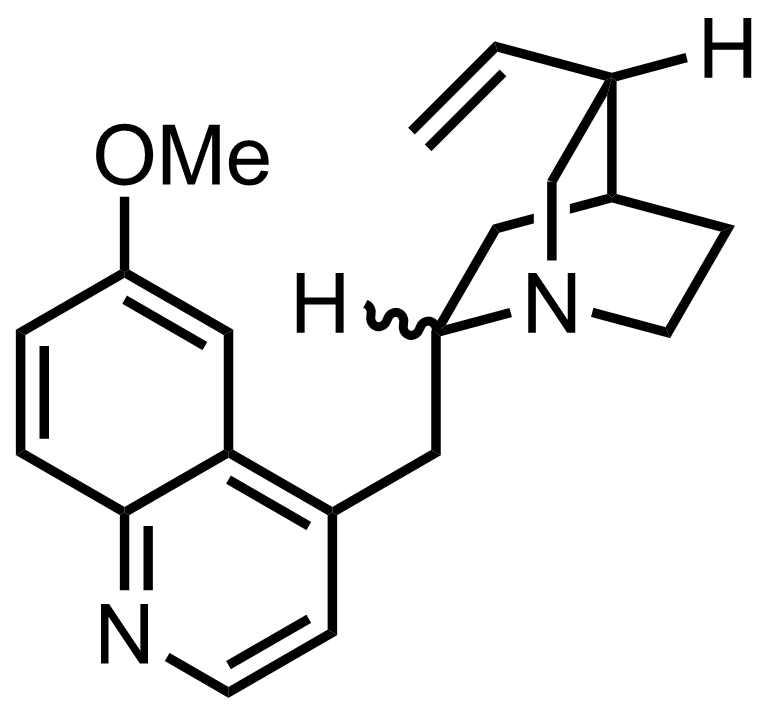


NaN3
PPA
120 °C, 30 min, 63%
See the Schmidt Reaction
"Also isolated was 13 % of the enamine regioisomer."

Rh/Al2O3,
HCl,
H2
EtOH, H2O
RT, 75 min, 100%

NaOAc,
N2O4
CCl4
-70 to 0 °C, 30 min, 100%

125 °C, 60 min, 49%

CH2N2
Et2O
RT, 15 min, 100%
 +
+

i-Pr2NLi
THF
-78 °C to RT, 60 min, 78%

i-Bu2AlH
PhMe
-78 °C, 80 min, 85%
"The enantiomers were resolved with dibenzoyl-(+)-tartaric acid."


NaOAc
AcOH, PhH
Reflux, 14 h, 80%

KOt-Bu,
O2
DMSO, t-BuOH
RT, 20 min, 32%
"Quinine was selectively cristallized (as a tartrate salt) from the resulting four diastereoisomers."

