Synthesis of Coccinelline
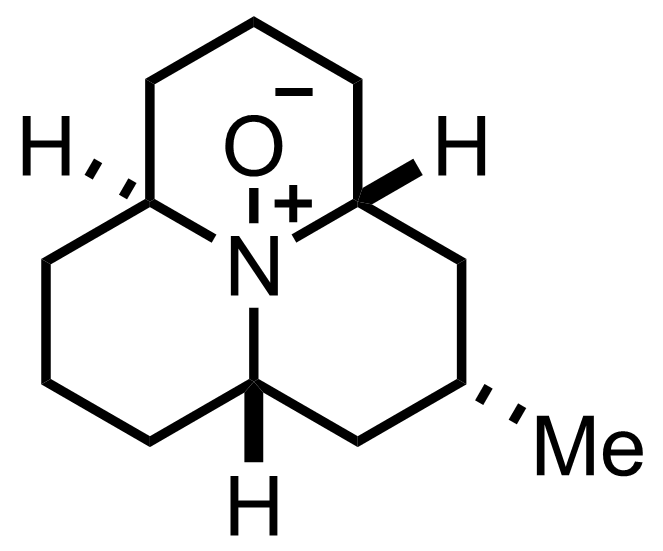
C13H23NO
| Principal investigator | Robert V. Stevens |
|---|---|
| Publication year | 1979 |
| Synthesis type | Total |
| Number of steps | 11 (linear) |
| References |
Part 1 of 1
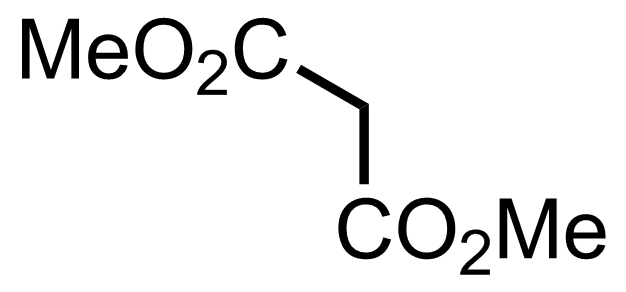 +
+
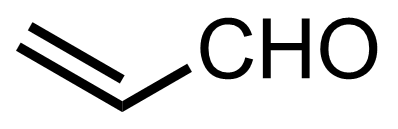
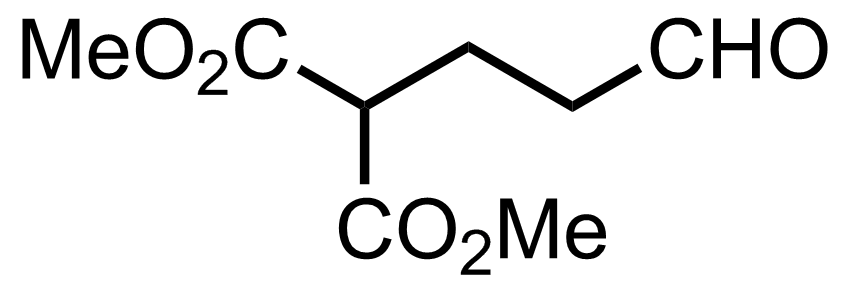
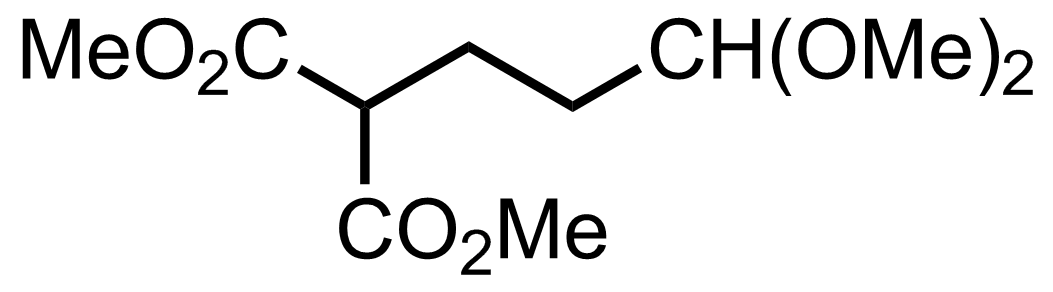
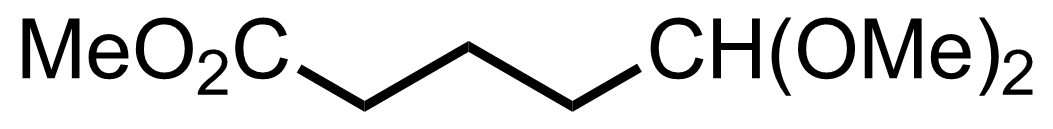
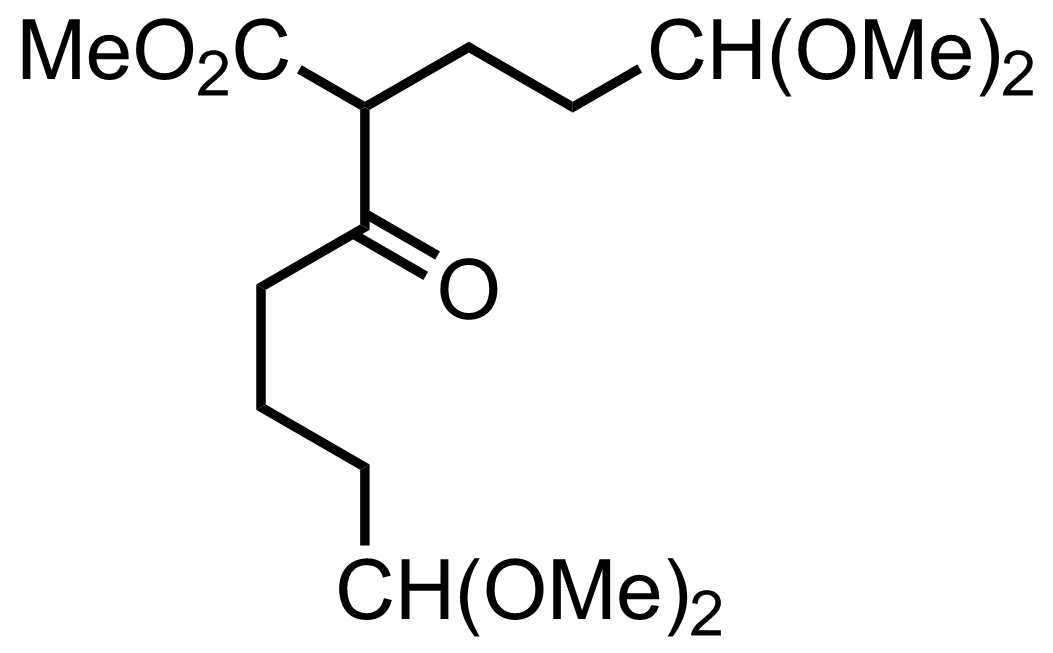
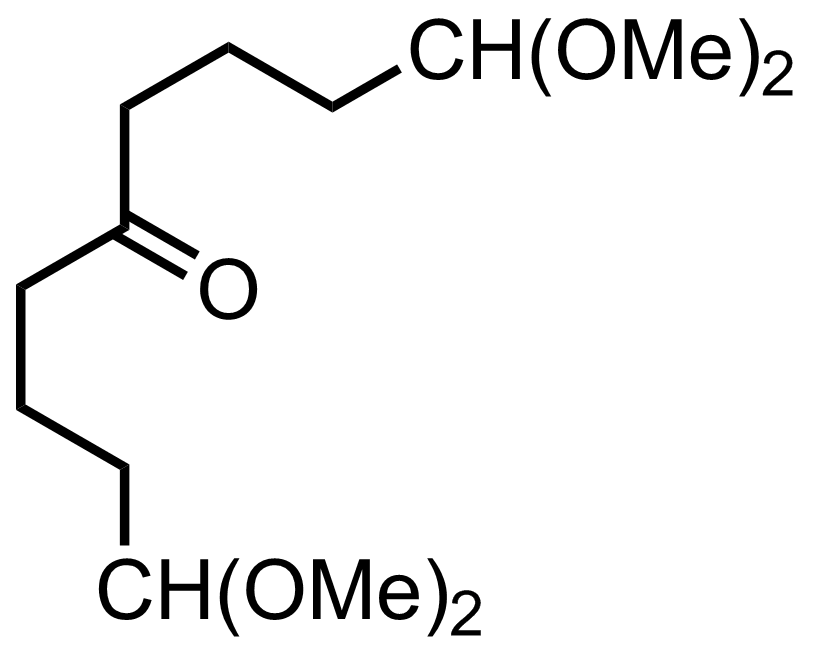
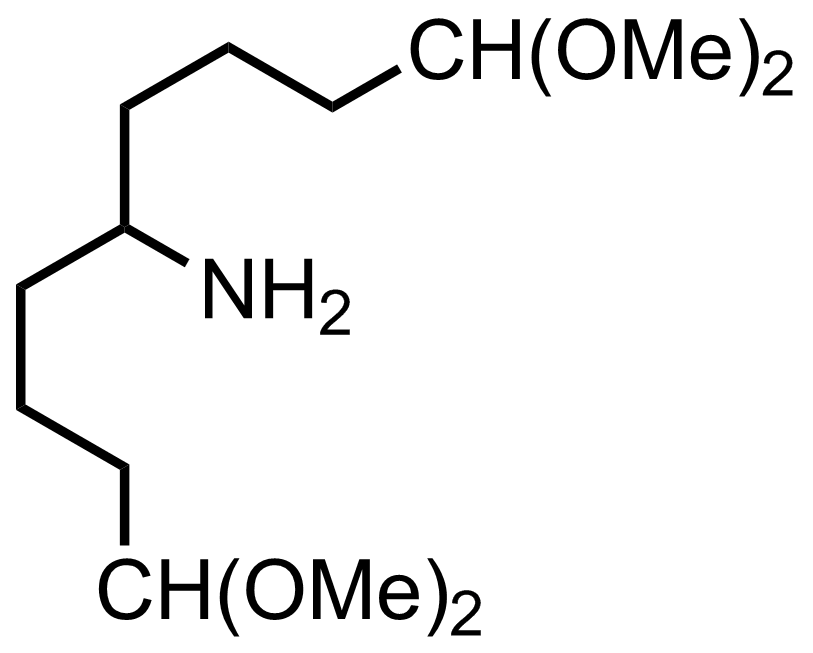 +
+
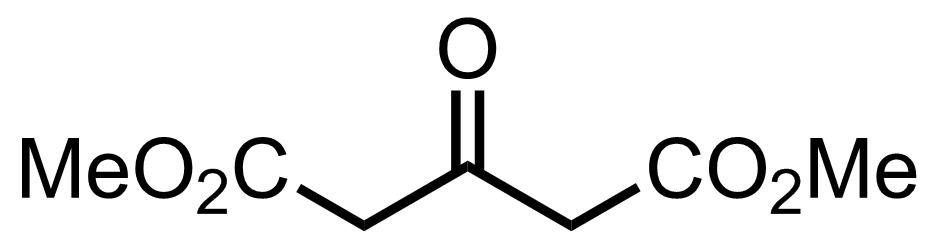
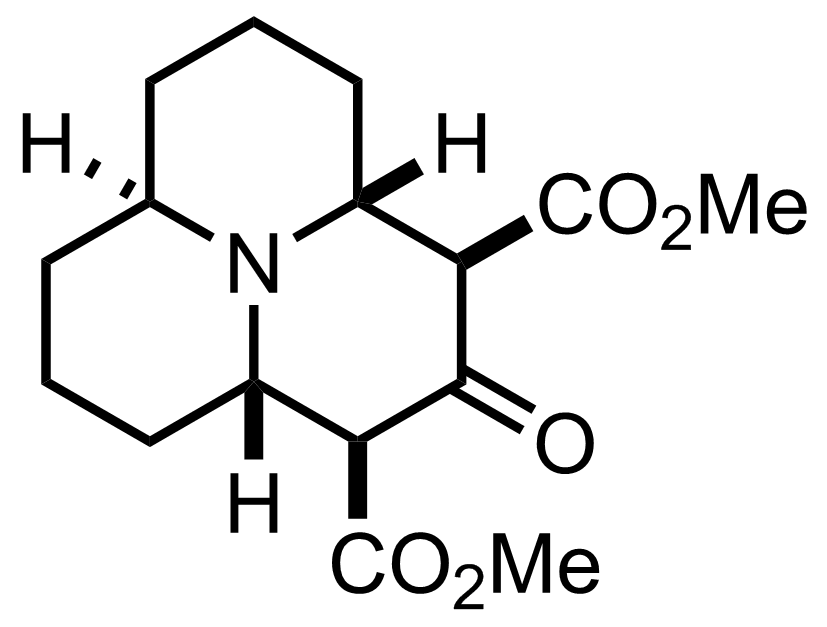
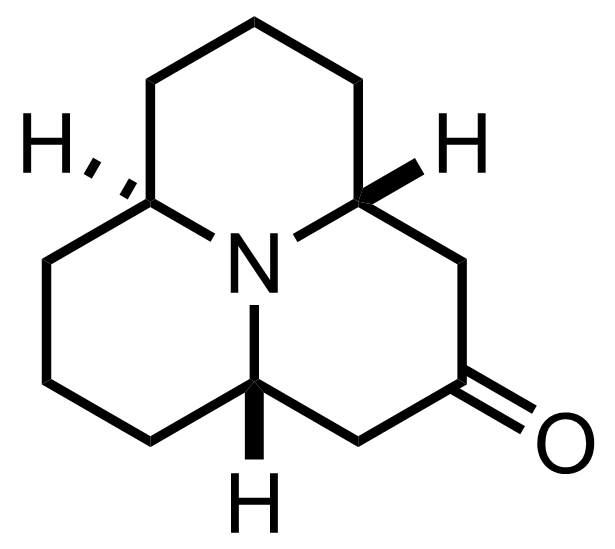



 +
+
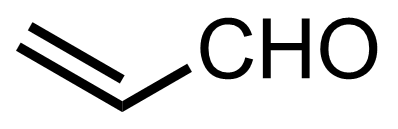

TsOH,
HC(OMe)3
MeOH
RT, 12 h, 98%




NH4OAc,
NaBH3CN
3 Å MS
MeOH
RT, 24 h, 99%
 +
+




Pd/C,
H2
1 atm
MeOH
RT, 8 h

mCPBA
CH2Cl2
0 °C, 8 h, 64% (2 steps)

