Synthesis of Oseltamivir
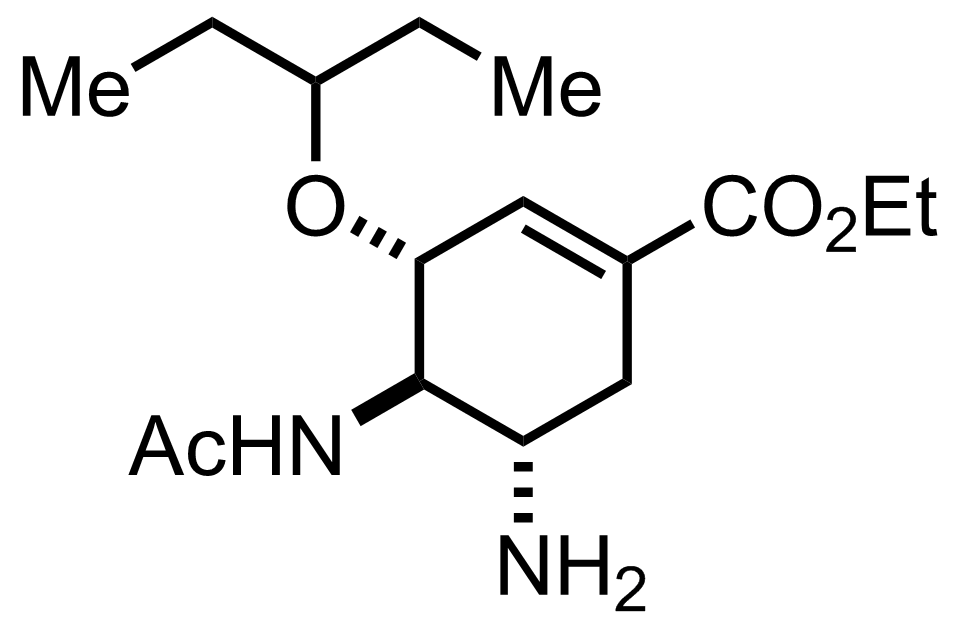
C16H28N2O4
| Principal investigator | Barry M. Trost |
|---|---|
| Publication year | 2008 |
| Synthesis type | Total |
| Number of steps | 9 (linear) |
| References |
Part 1 of 1

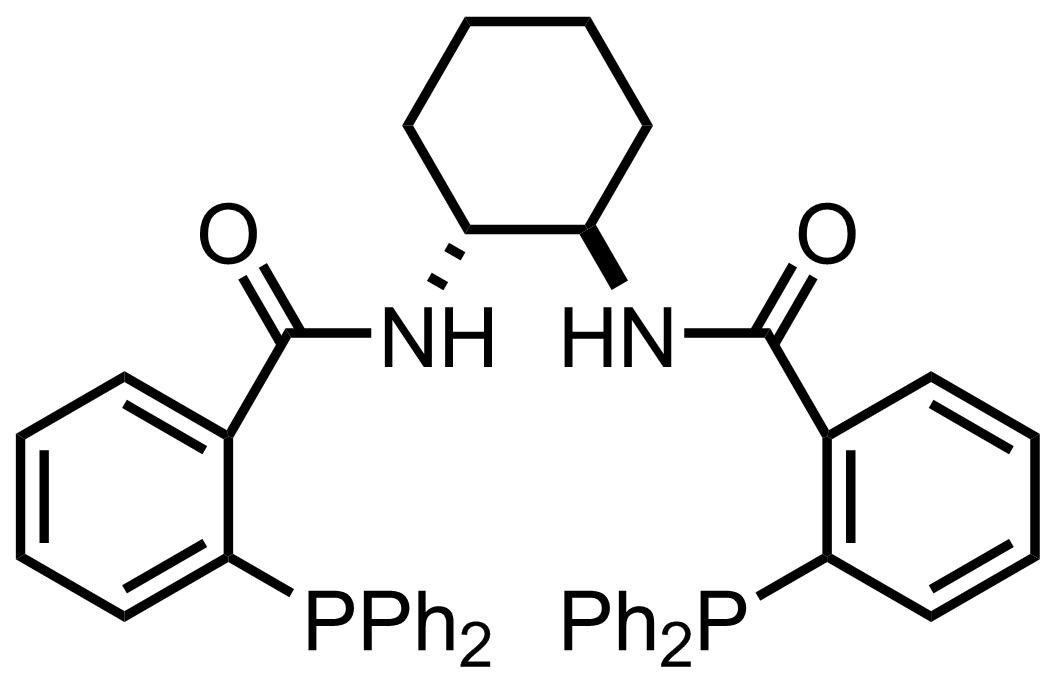
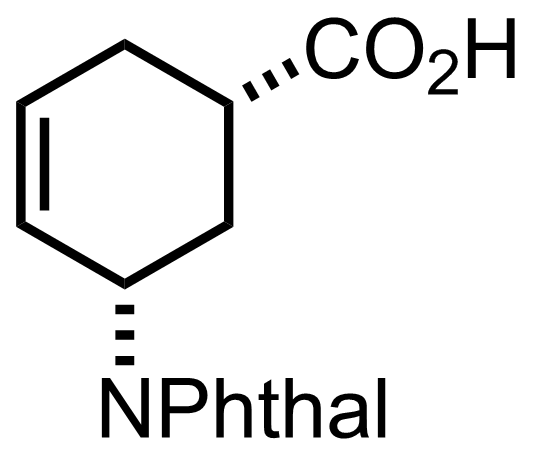
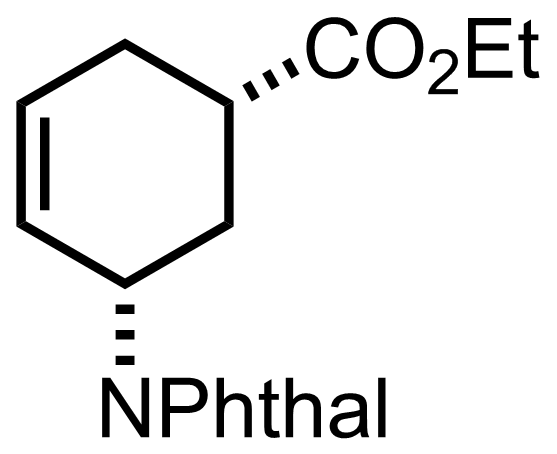
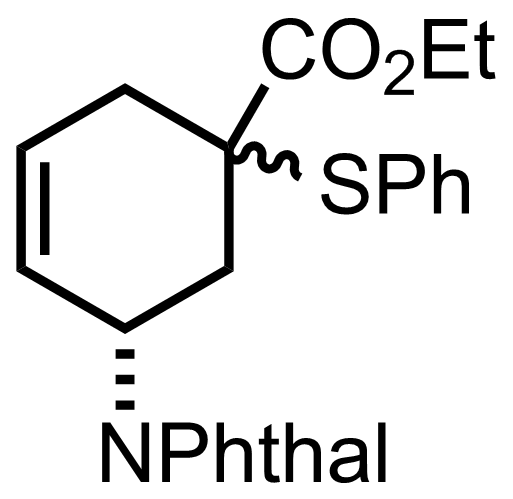
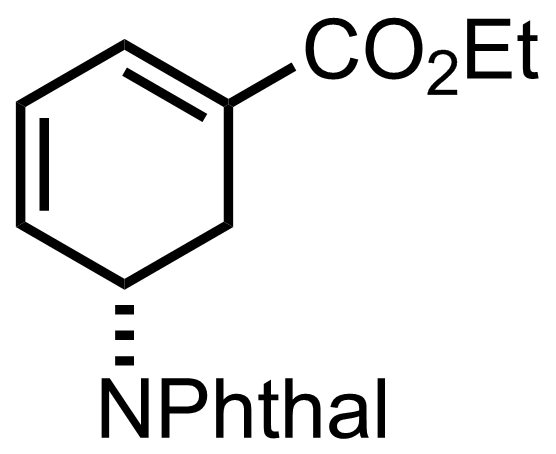
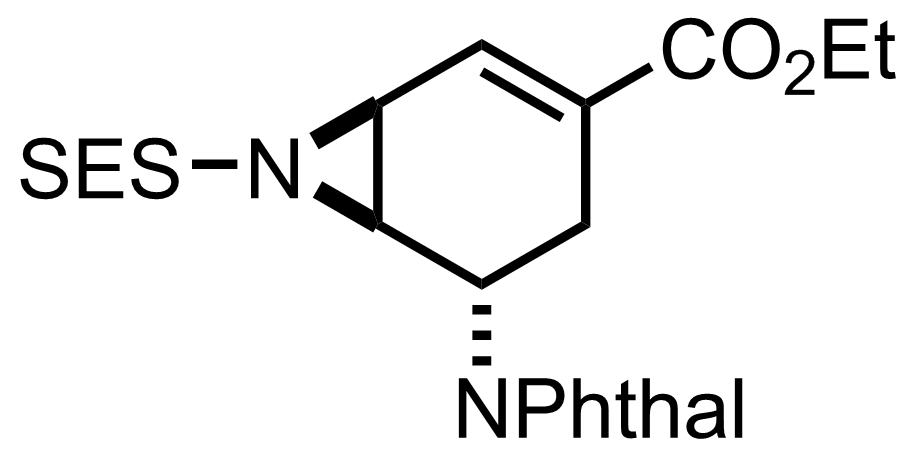
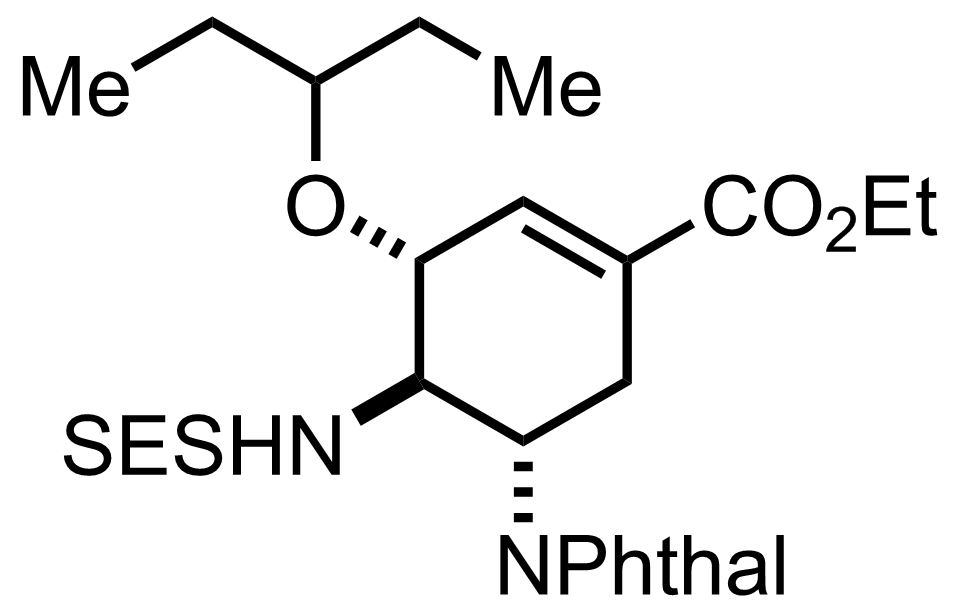





(n3-C3H3PdCl)2,
Trimethylsilylphthalimide
THF
40 °C, 8.5 h


PhSSO2Ph,
TMS2NK
THF
-78 °C to RT, ON, 94%

NaHCO3,
mCPBA,
DBU
PhMe
0 to 60 °C, 7 h, 85%

TMS(CH2)2SO2NH2,
PhI(OPiv)2,
Rh2(esp)2,
MgO
PhCl
0 °C to RT, 4 h, 86%

3-Pentanol,
BF3.OEt2
75 °C, 15 min, 65%

DMAP,
Ac2O
µW
Pyr
150 °C, 60 min, 84%

n-Bu4N+ F-
THF
RT, 30 min, 95%

NH2NH2
EtOH
68 °C, 10 h, 100%

