Synthesis of Oseltamivir
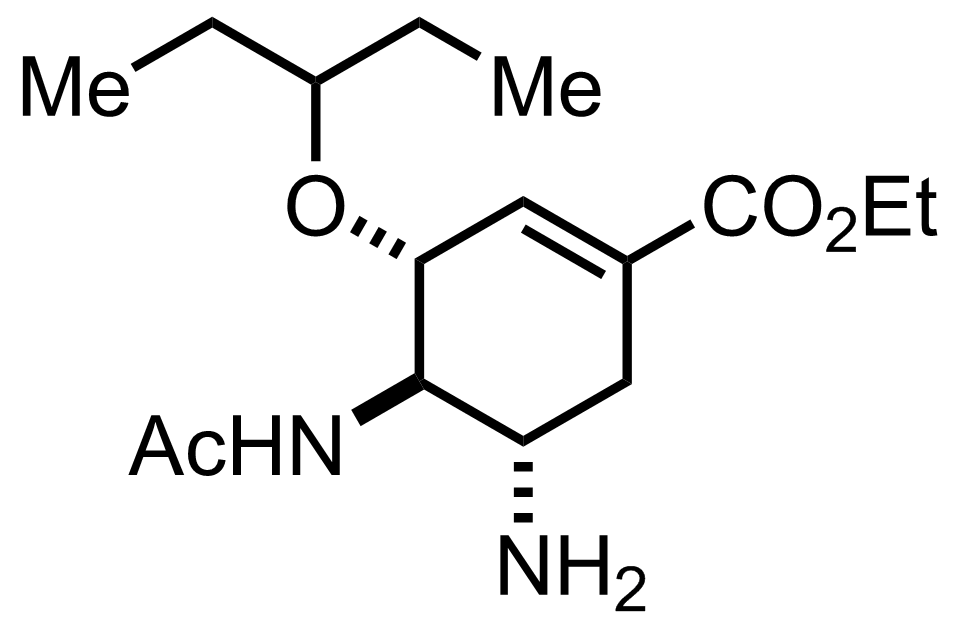
C16H28N2O4
| Principal investigator | Yujiro Hayashi |
|---|---|
| Publication year | 2009 |
| Synthesis type | Total |
| Number of steps | 8 (linear) |
| References |
Part 1 of 1
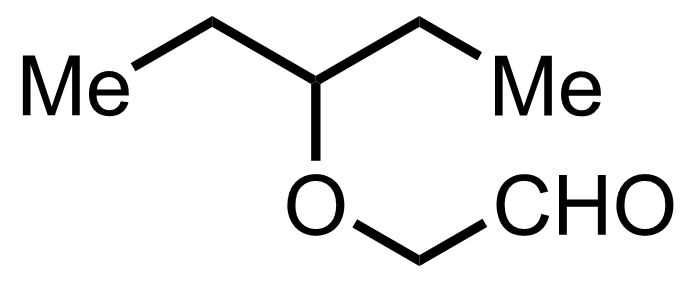 +
+
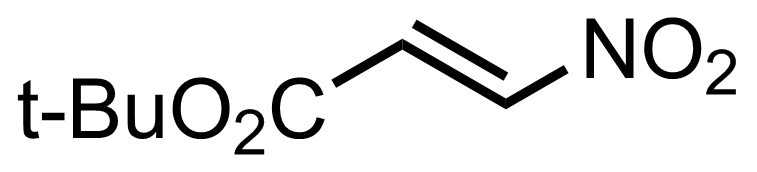 +
+
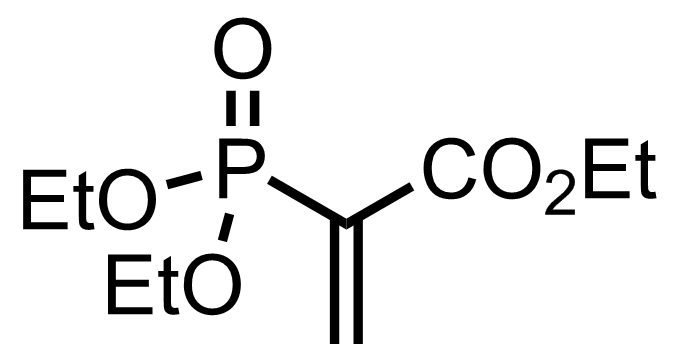
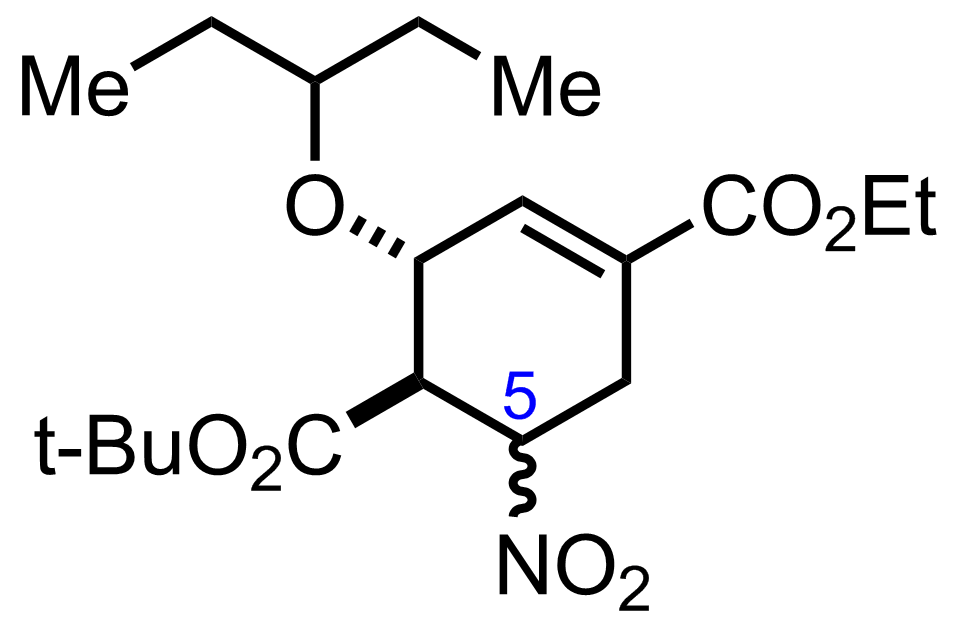
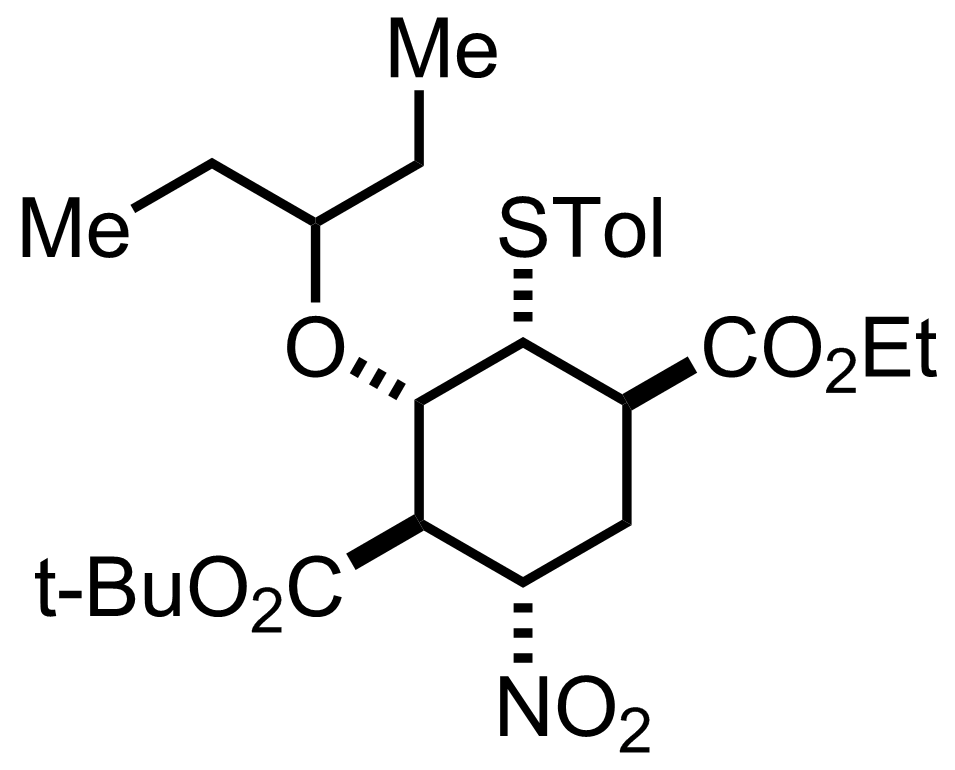
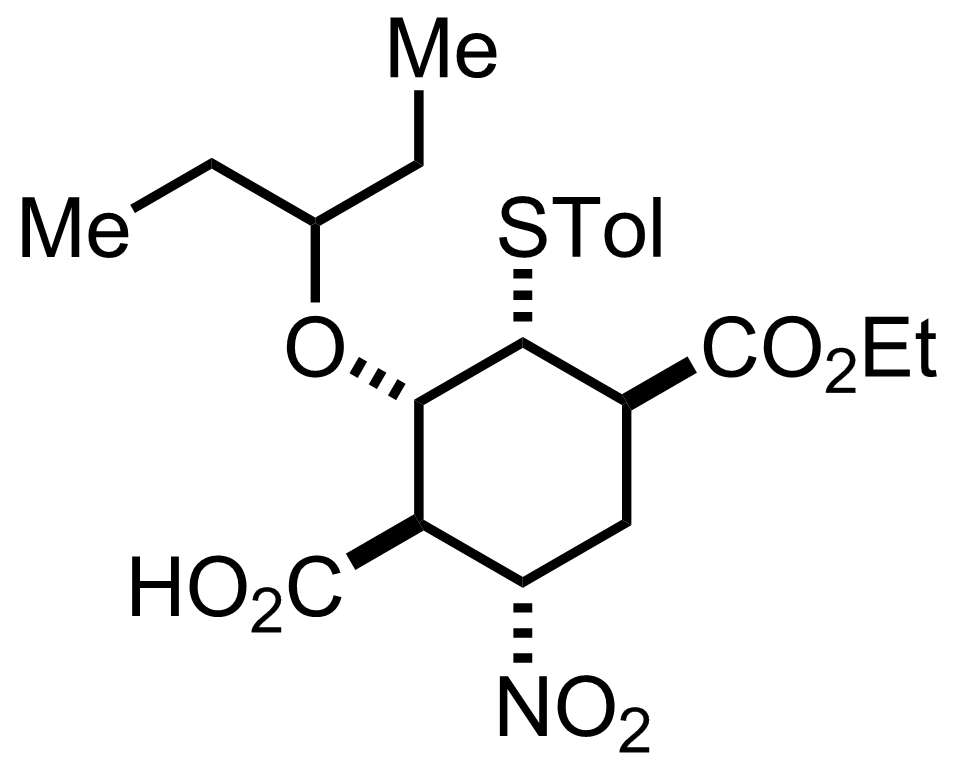
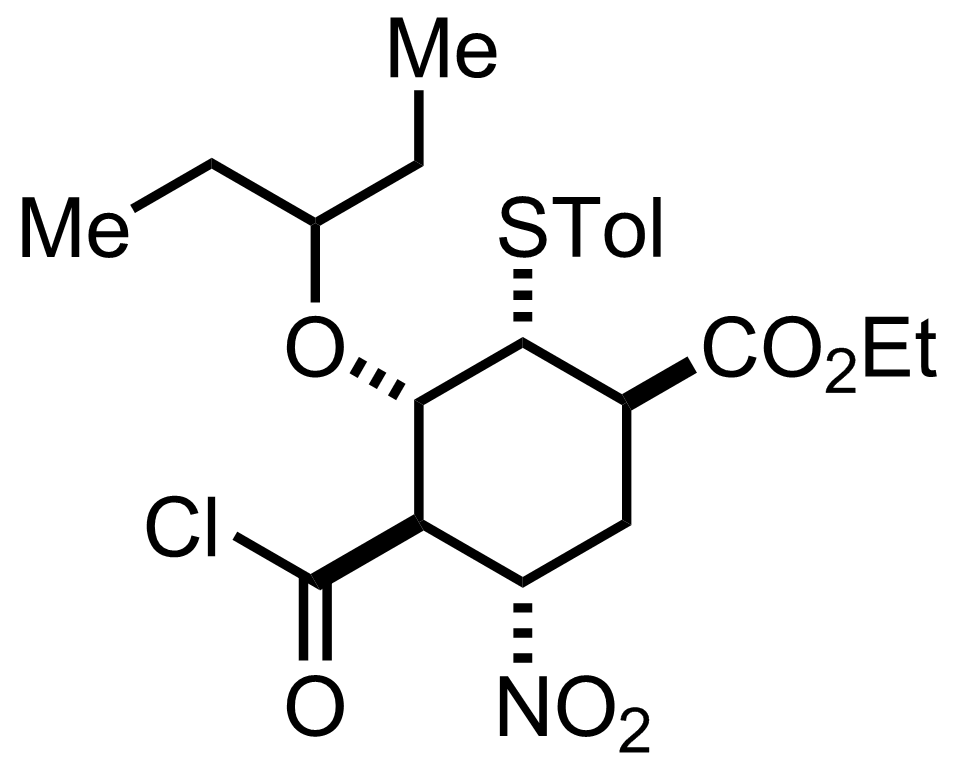
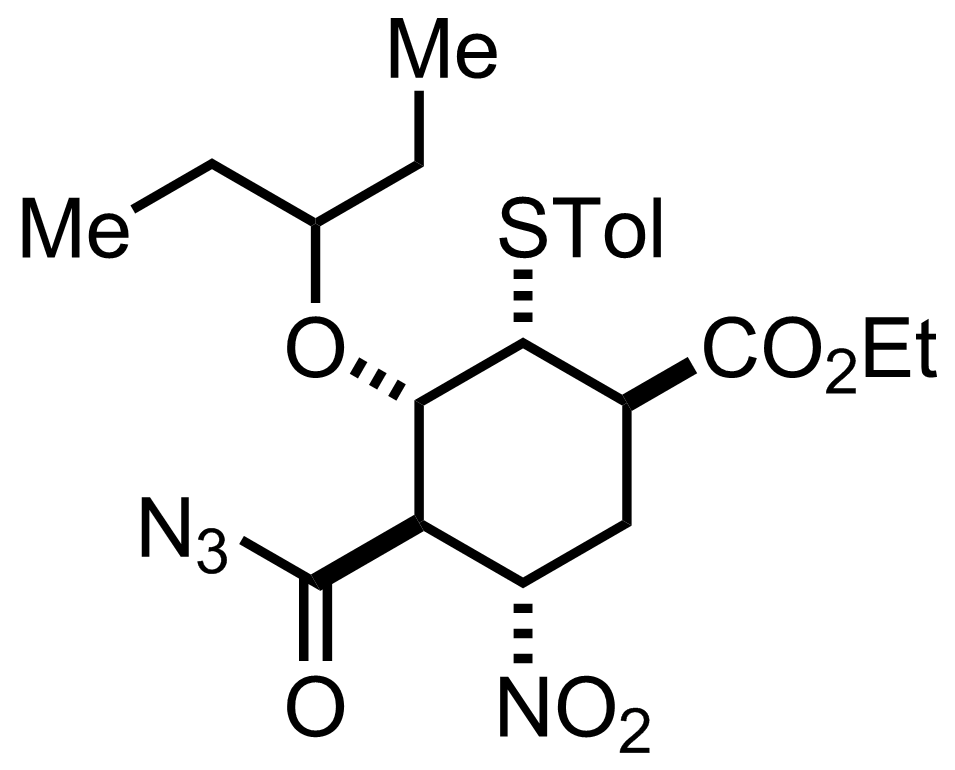
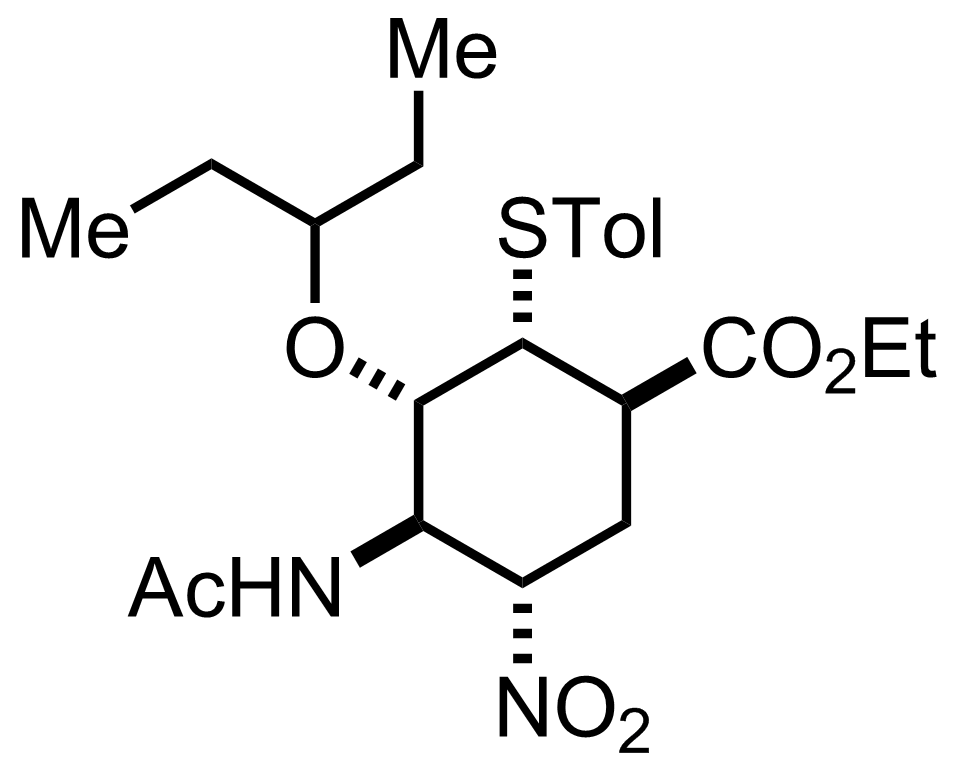

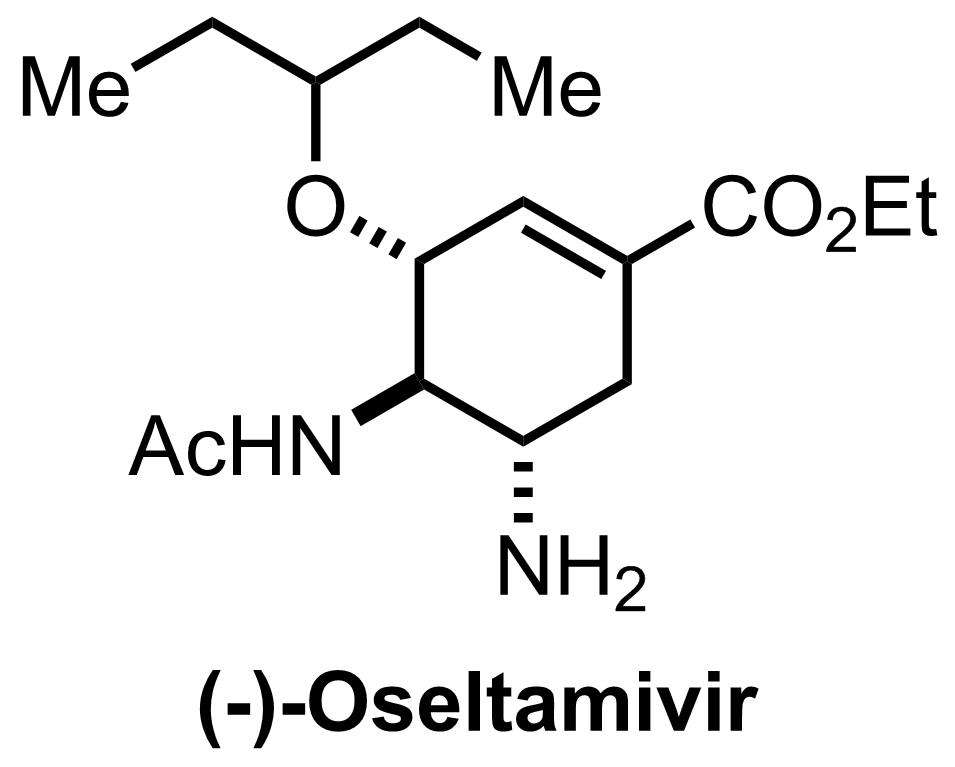
 +
+
 +
+



CF3CO2H
CH2Cl2
RT, 2 h

DMF,
(COCl)2
CH2Cl2
0 °C to RT, 60 min

NaN3
Acetone, H2O
0 °C, 20 min


Zn,
TMSCl
EtOH
70 °C, 2 h

K2CO3,
NH3
EtOH
0 °C to RT, 6 h, 82% (6 steps)


