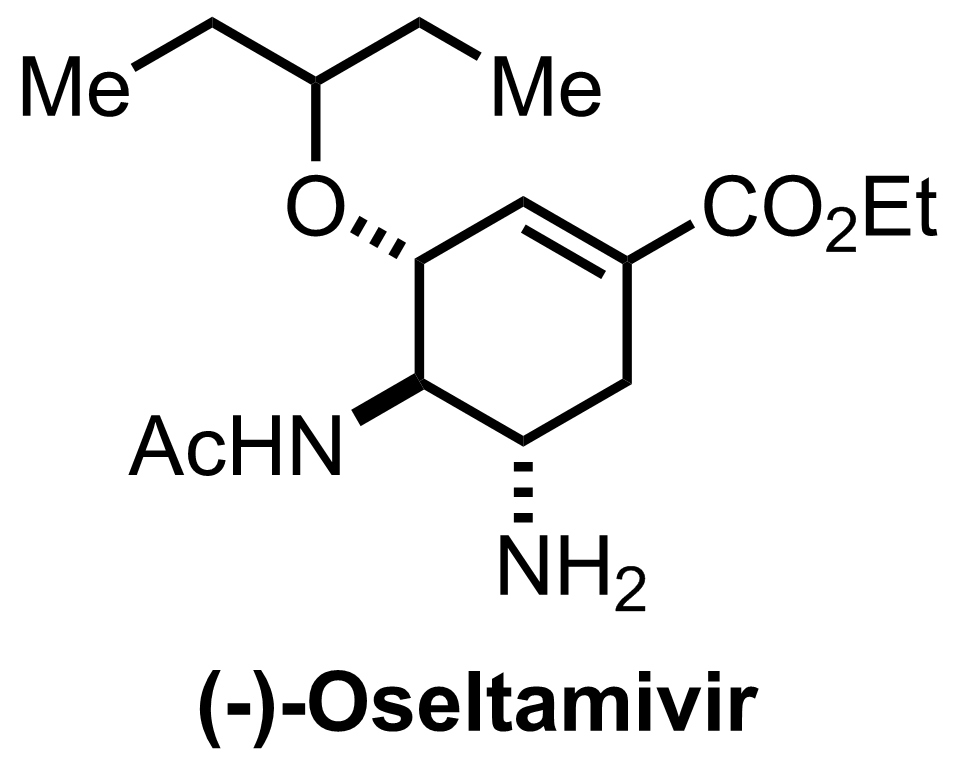
Synthesis of Oseltamivir
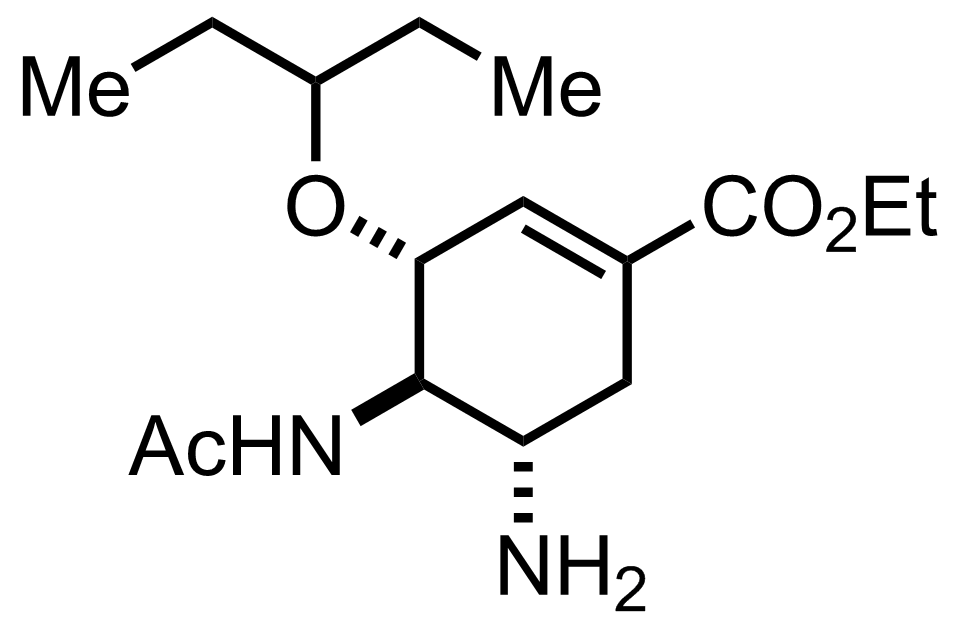
C16H28N2O4
| Principal investigator | Masakatsu Shibasaki |
|---|---|
| Publication year | 2007 |
| Synthesis type | Total |
| Number of steps | 14 (linear) |
| References |
Part 1 of 1
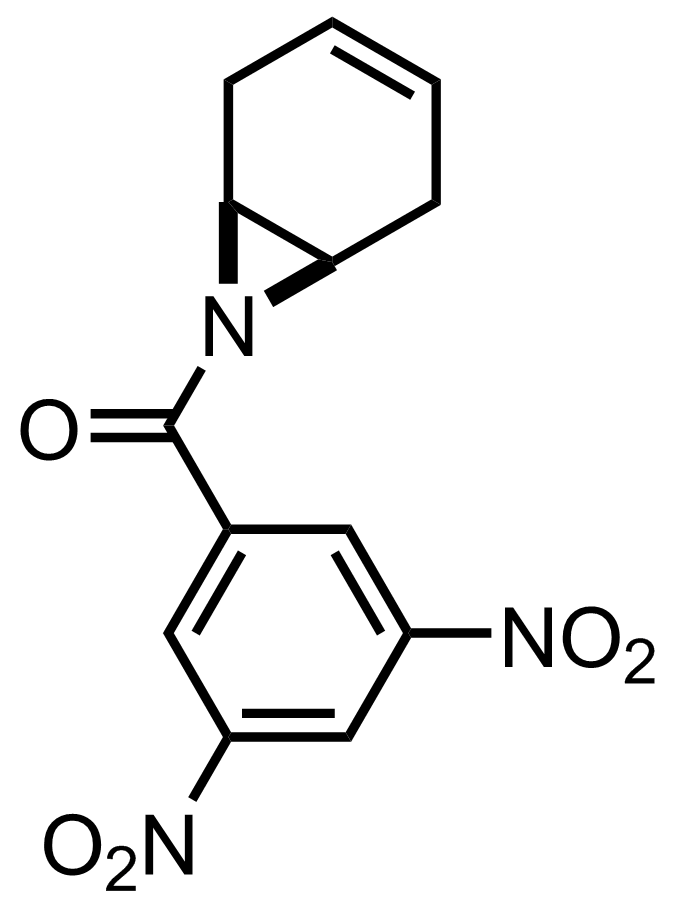
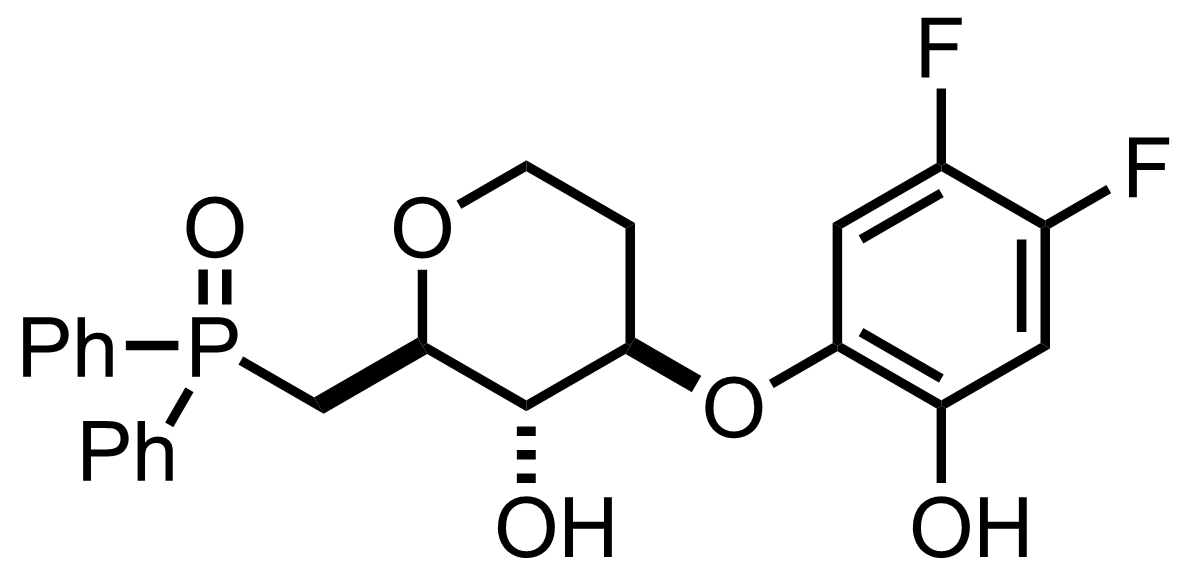

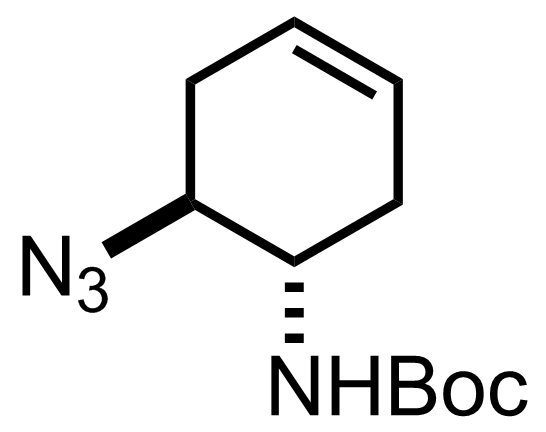


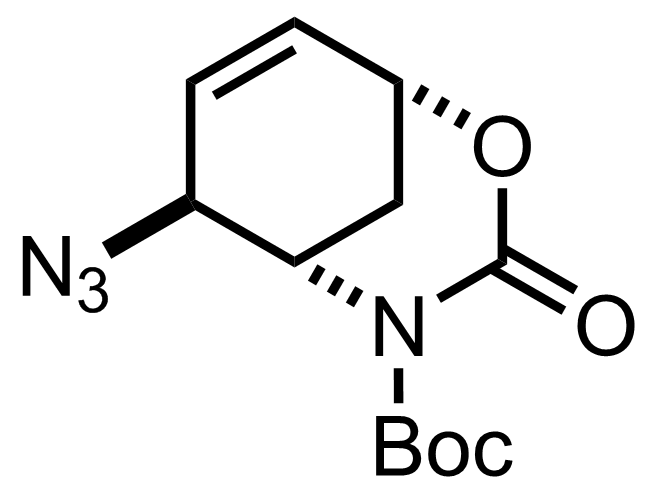

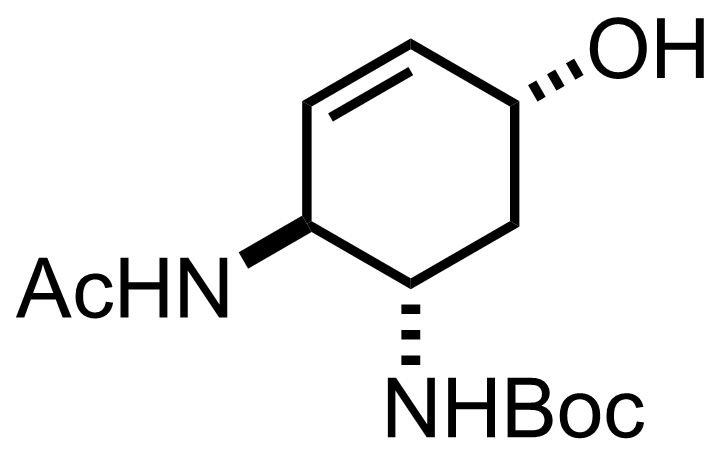
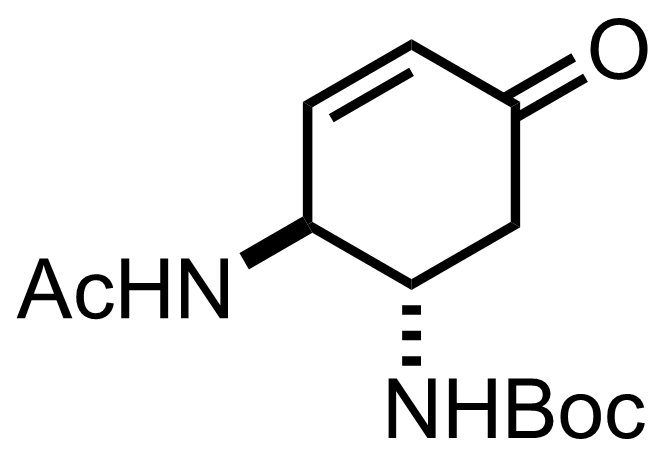

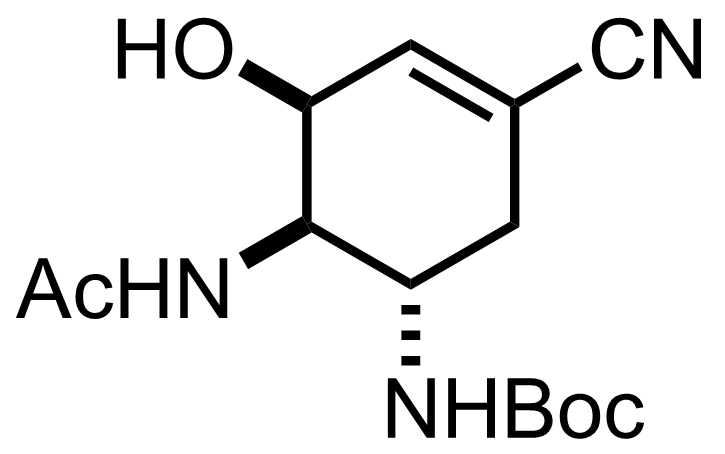


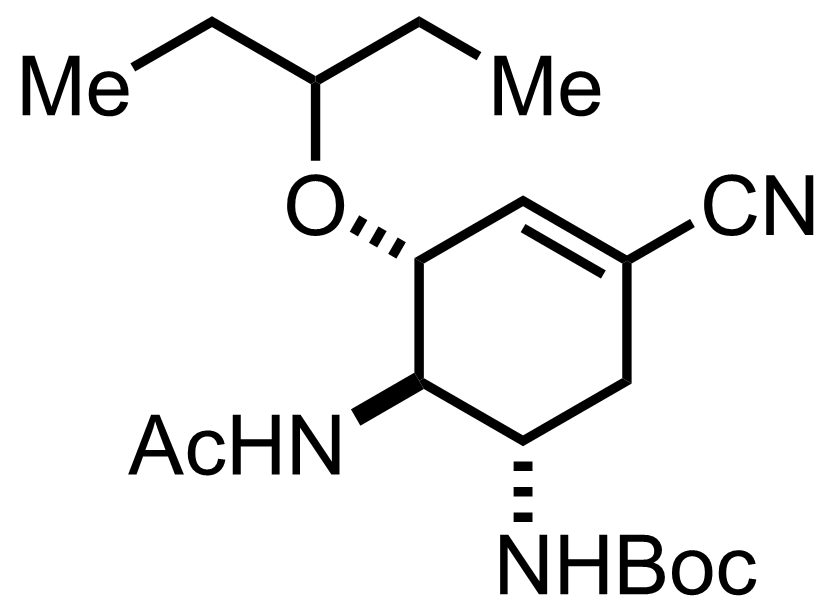


TMSN3,
Y(Oi-Pr)3,
2,6-Dimethylphenol
EtCN
RT, 12 h, 94%
"Cristallized to >99:01 er (72 % yield)."

DMAP,
NaOH,
Boc2O
H2O, MeCN
RT, 2 h, 98%

K2CO3,
I2,
KI
CHCl3
60 °C, 14 h

DBU
CHCl3
4 °C to RT, 4 h, 85% (2 steps)

Boc2O,
Et3N,
DMAP
CH2Cl2
4 °C to RT, 2 h, 99%

2,6-Lutidine,
AcSH
CHCl3
60 °C, 19 h, 83%

Cs2CO3
n-BuOH
RT, 16 h, 86%


(EtO)2P(O)CN,
LiCN
THF
-20 °C, 7 h

Sealed tube
PhMe
140 °C, 40 min, 78% (2 steps)



BF3.OEt2,
3-Pentanol
5 Å MS
-20 °C, 75 min, 56%


