Synthesis of Oseltamivir
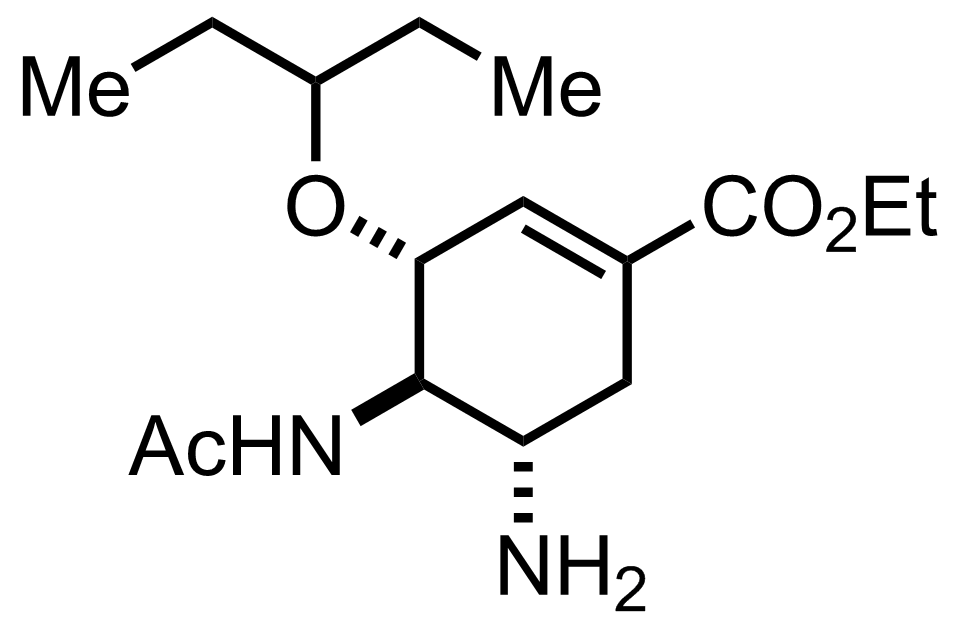
C16H28N2O4
| Principal investigator | John C. Rohloff |
|---|---|
| Publication year | 1998 |
| Synthesis type | Total |
| Number of steps | 12 (linear) |
| References |
Part 1 of 1
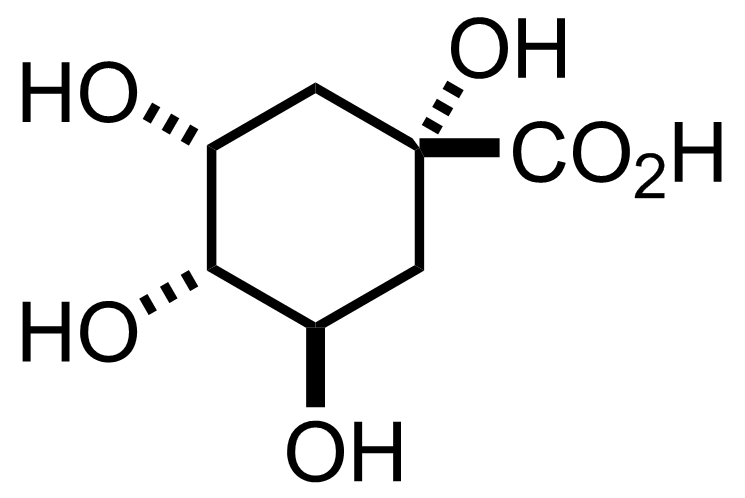
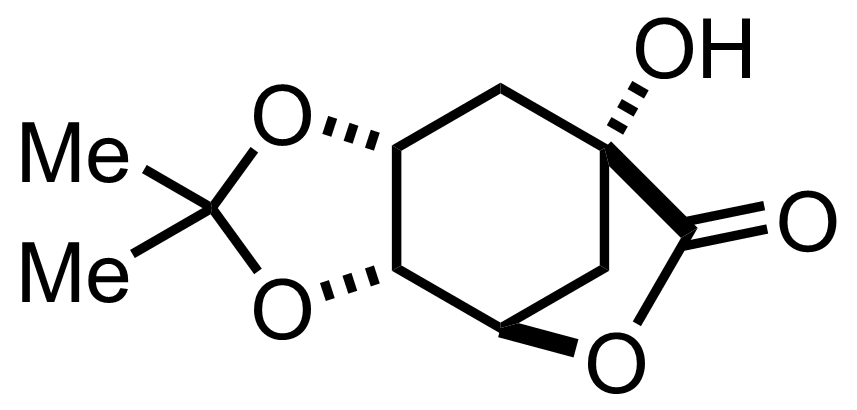
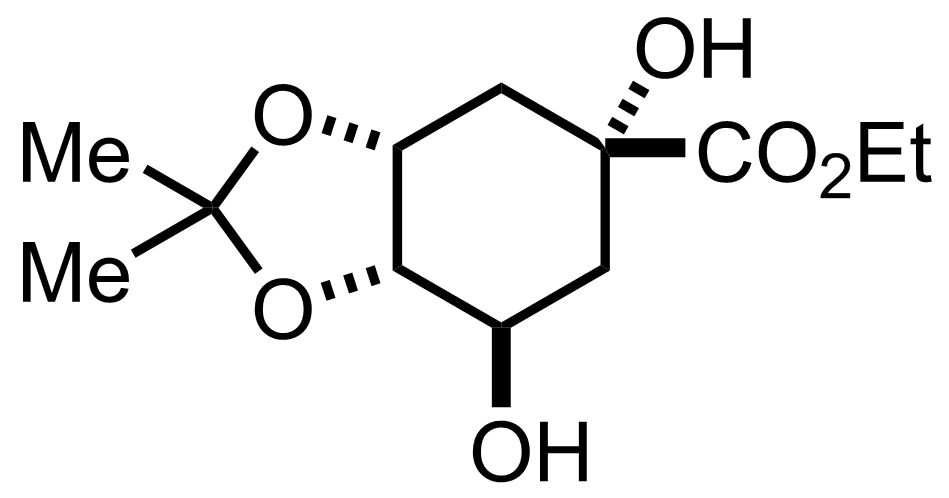
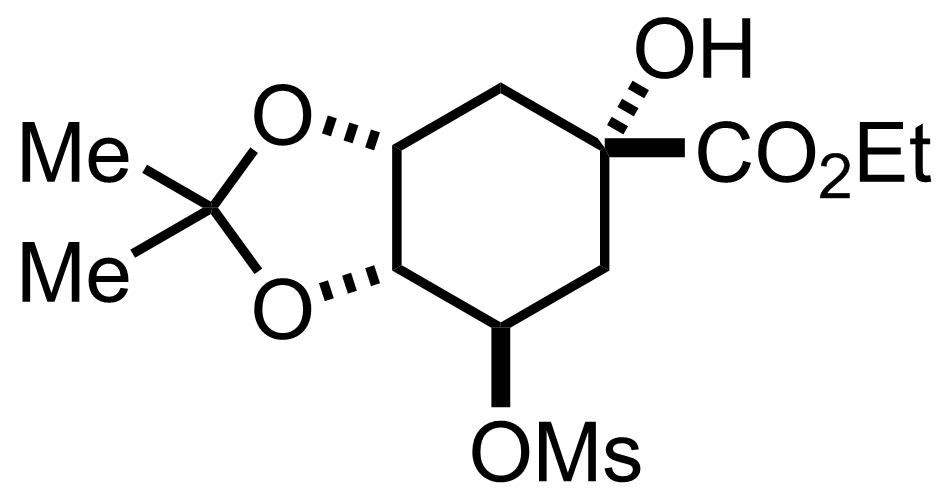
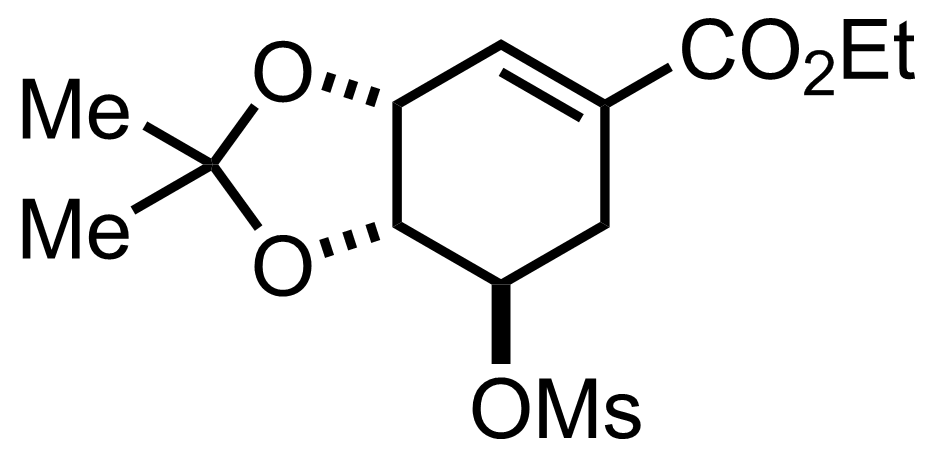
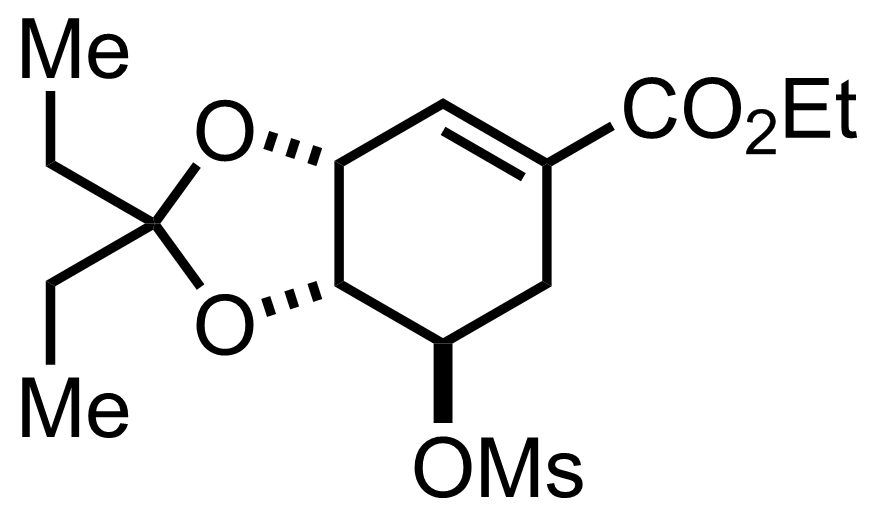
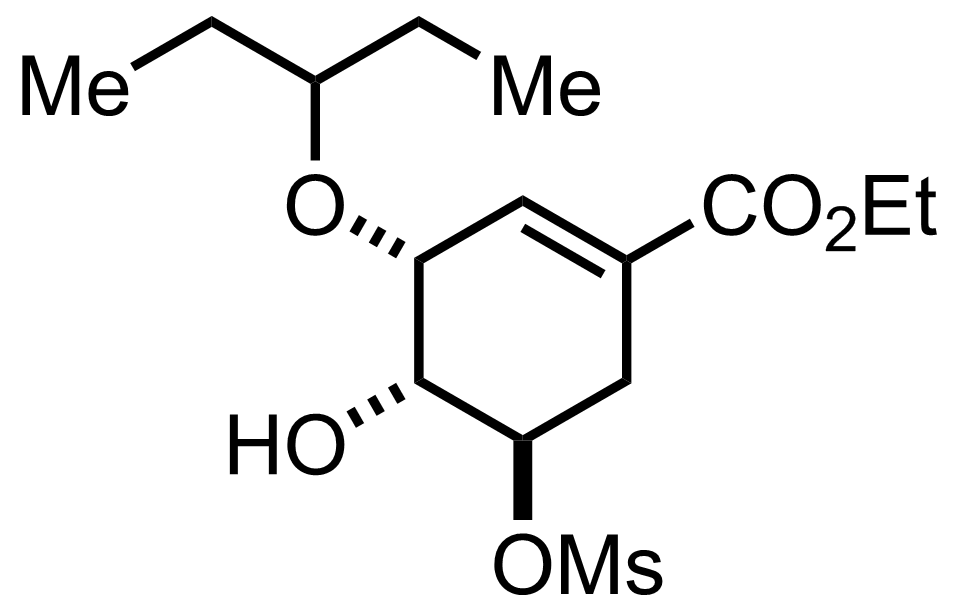
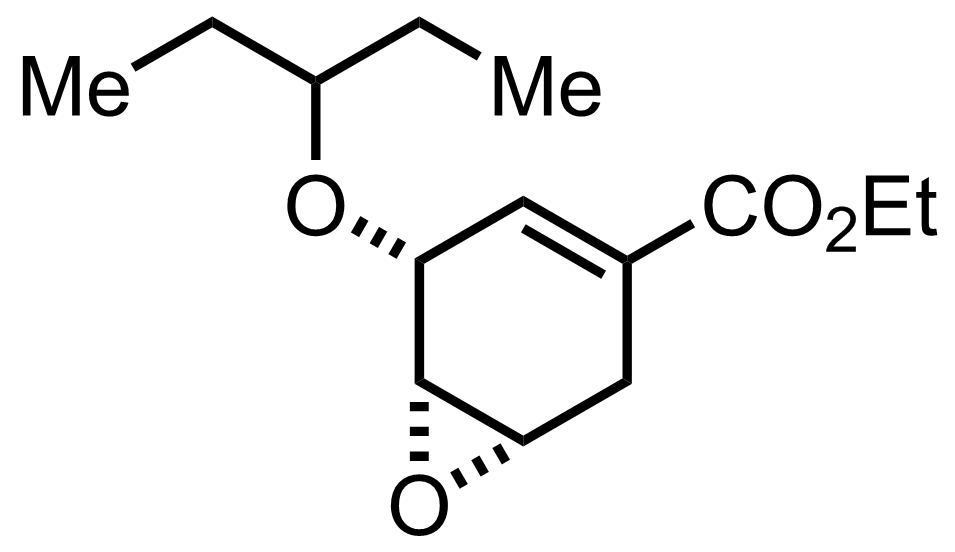
 +
+
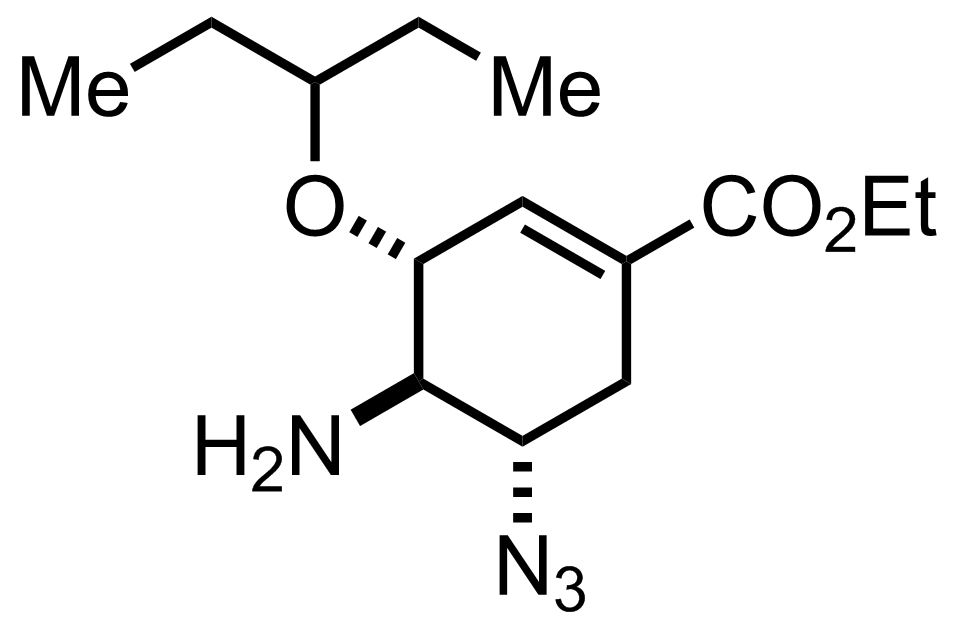
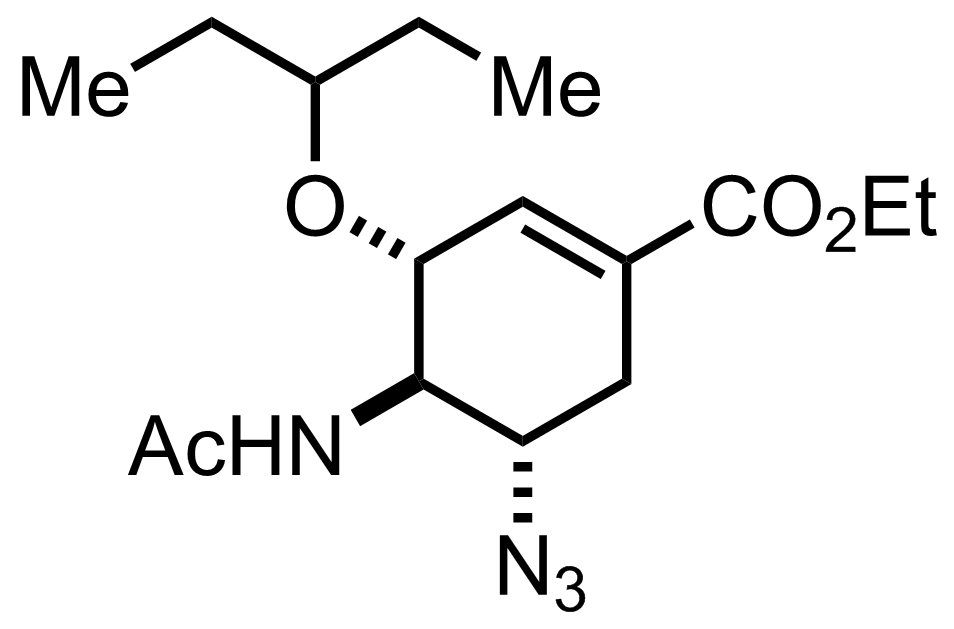
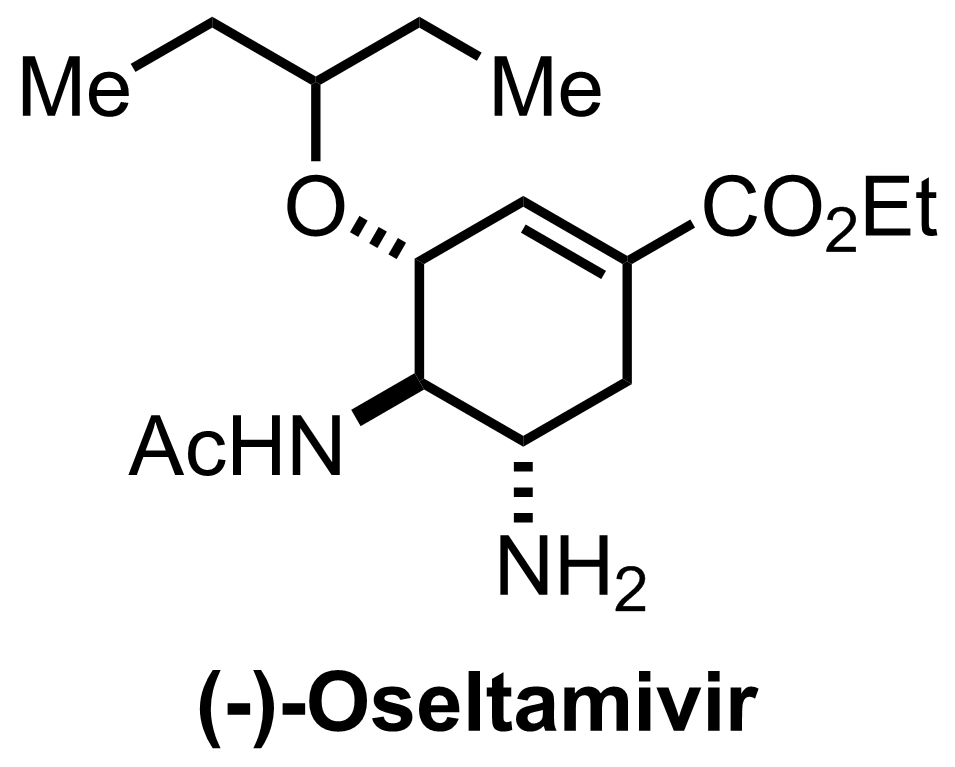

TsOH,
2,2-Dimethoxypropane
Acetone
Reflux, 2 h

NaOEt
EtOH
RT, 2 h

Et3N,
MsCl
CH2Cl2
0-5 °C, 90 min, 69% (3 steps)

Pyr,
SO2Cl2
CH2Cl2
-30 to -20 °C, 3.5 h, 44%

HClO4,
3-Pentanone
H2O
40 °C, 4-20 h, 95%

BH3.Me2S,
TMSOTf
CH2Cl2
-20 to -10 °C, 42 min

KHCO3
EtOH, H2O
55 to 65 °C, 60 min, 64% (2 steps)

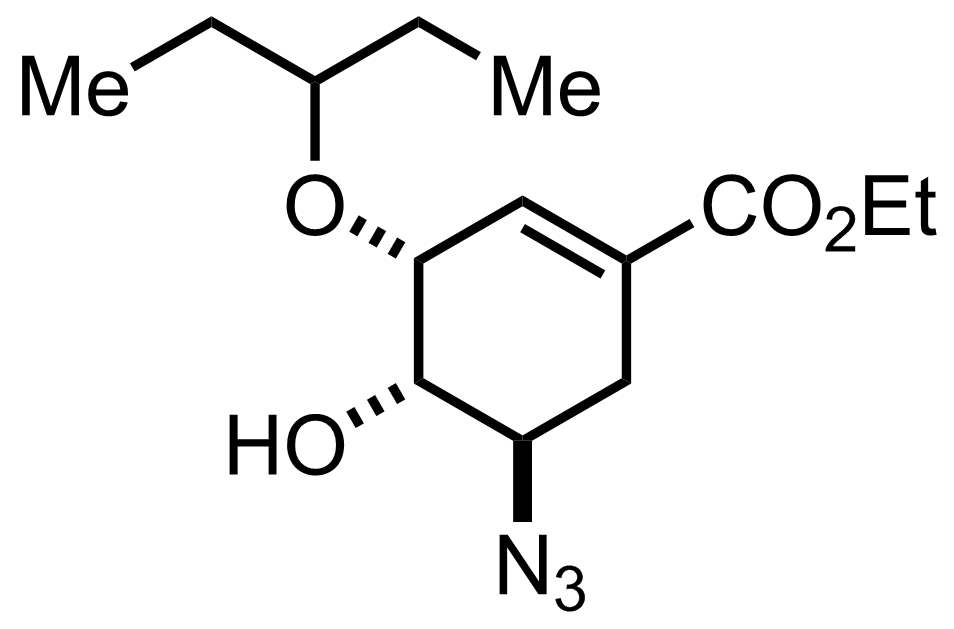
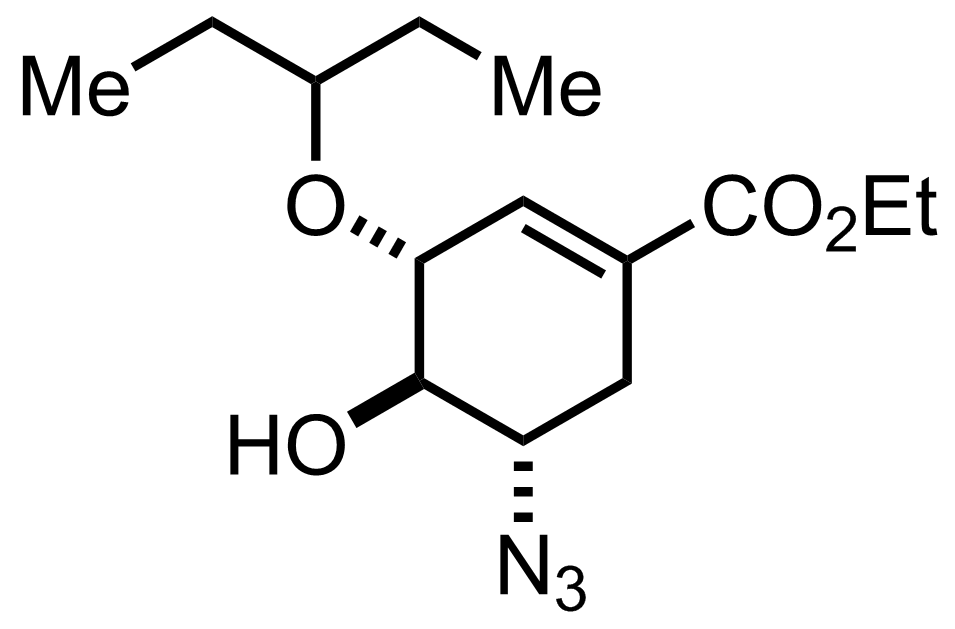
NaN3,
NH4Cl
EtOH, H2O
70-75 °C, 12-18 h, 86%
 +
+

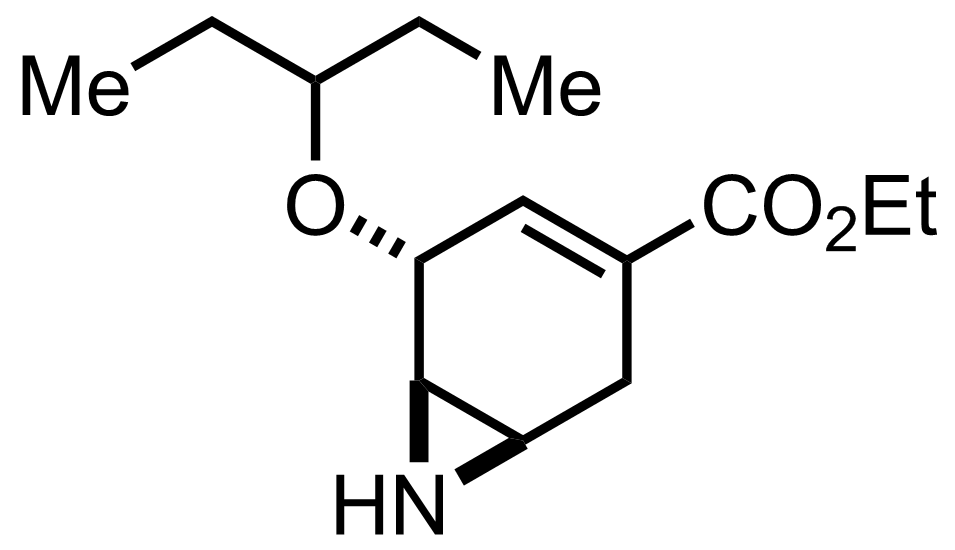
Me3P
MeCN
RT to 38 °C, 2 h

NaN3,
NH4Cl
DMF
70 to 80 °C, 12-18 h


H2,
Ni (Raney)
1 atm
EtOH
RT, 10-16 h, 71%

