Synthesis of Reserpine
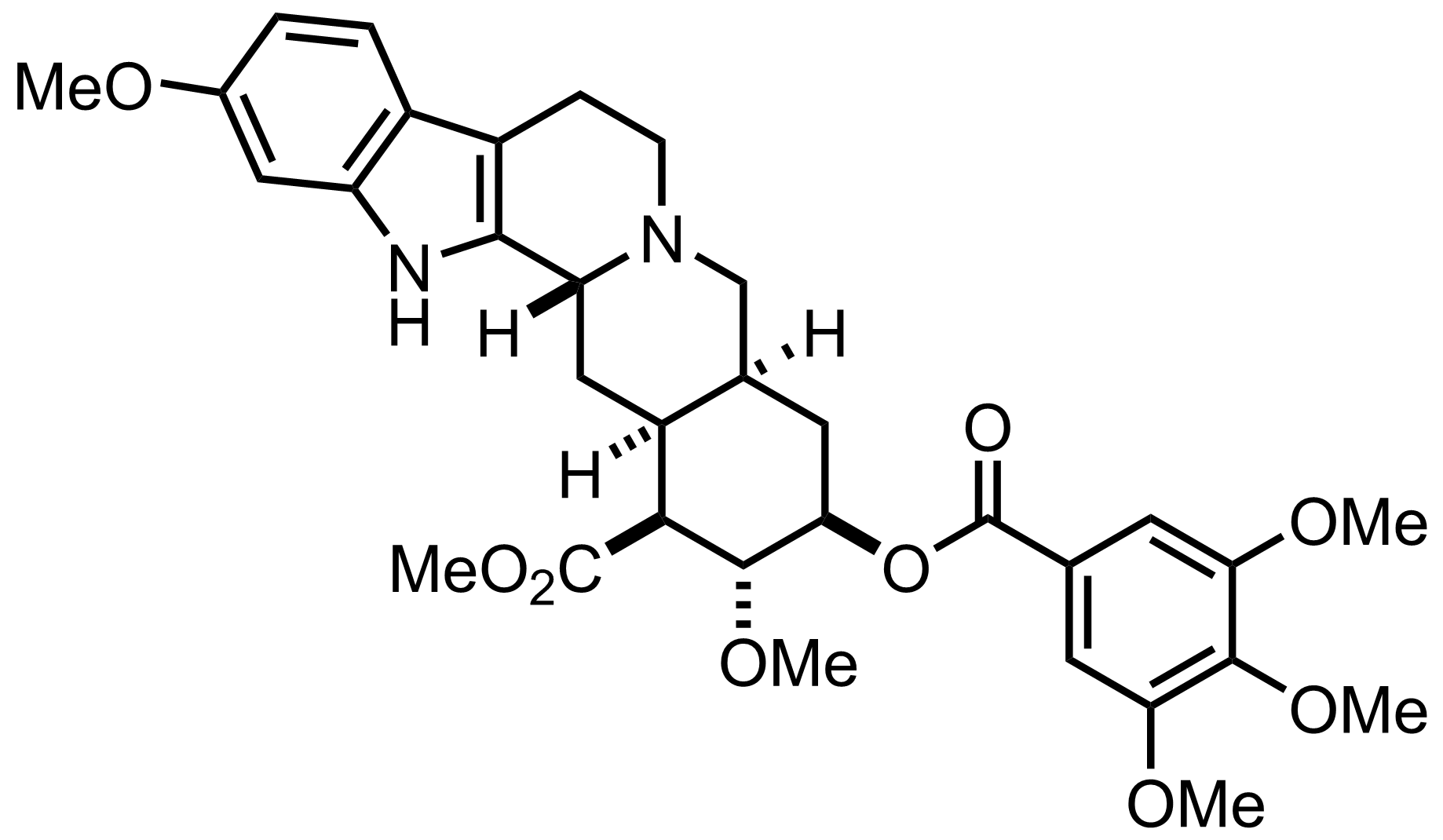
C33H40N2O9
| Principal investigator | Stephen Hanessian |
|---|---|
| Publication year | 1997 |
| Synthesis type | Total |
| Number of steps | 22 (linear) |
| References |
Part 1 of 1



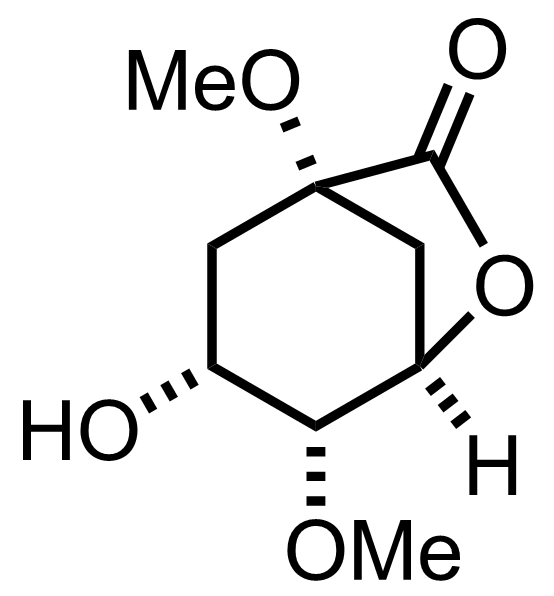
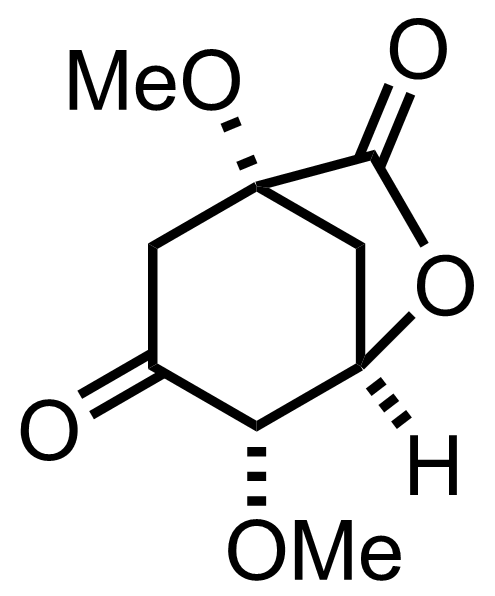
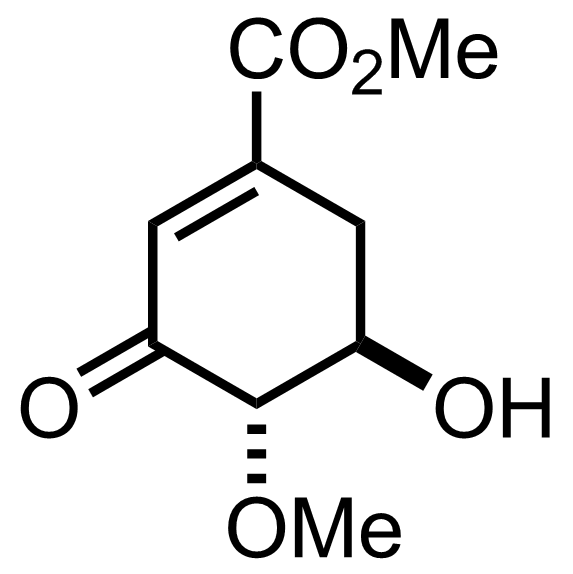
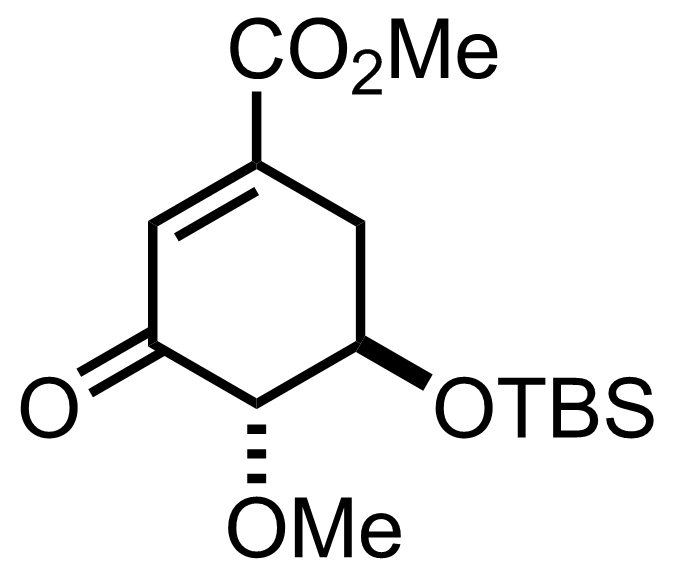
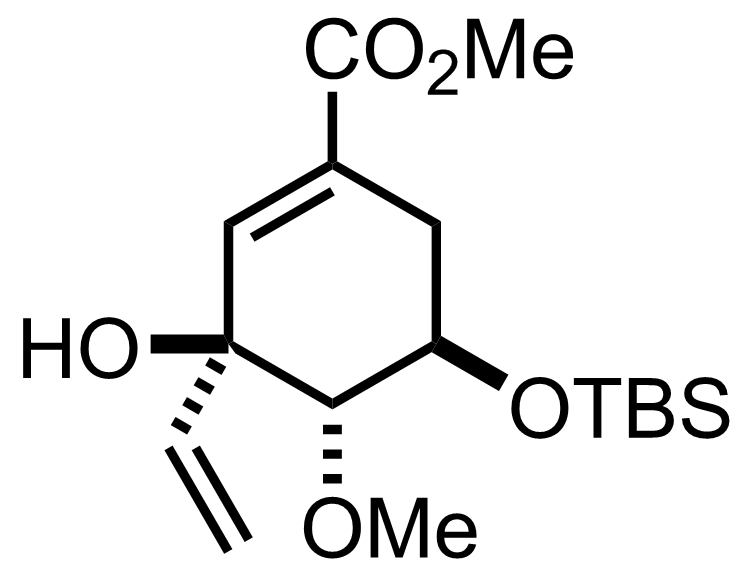 +
+
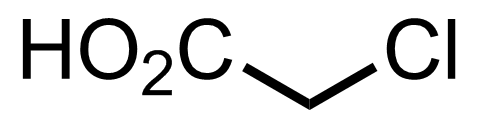
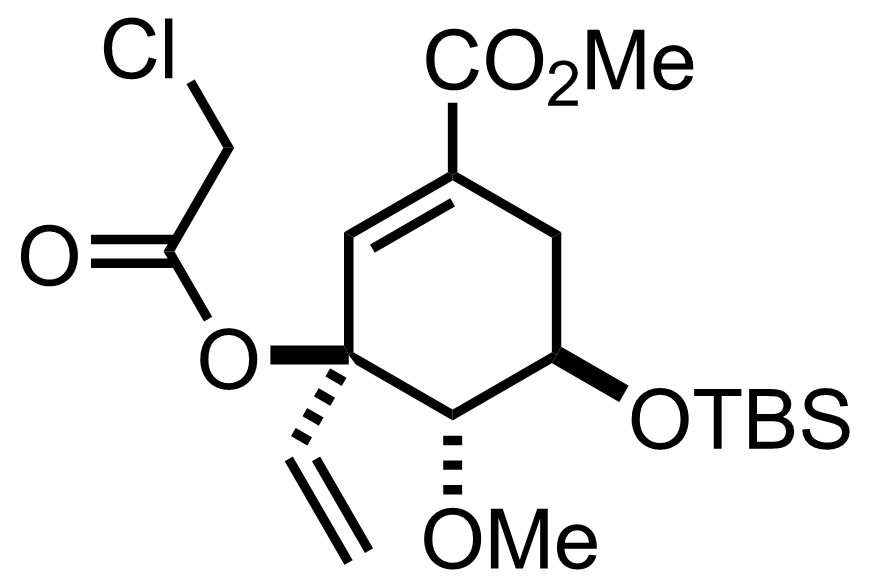



 +
+


 +
+
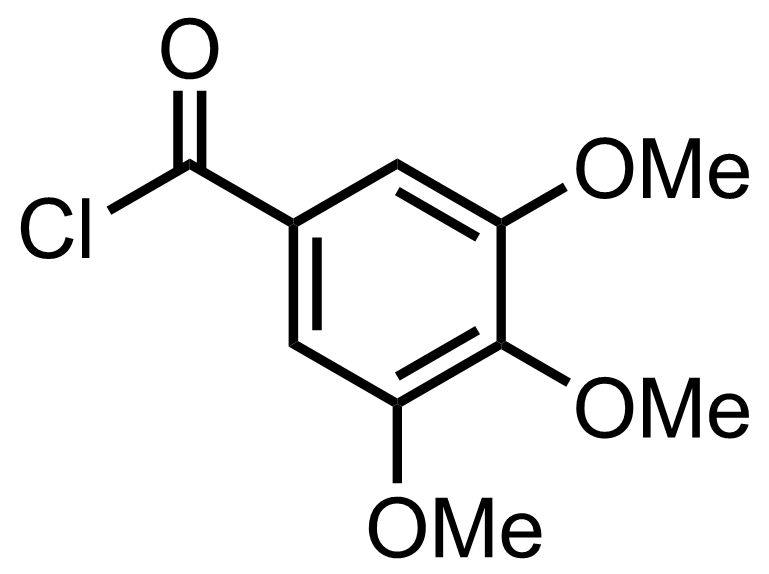


BnBr,
TsOH,
n-Bu2SnO
Dean-Stark
DMF, PhH
Reflux, 22 h, 81%


H2,
Pd(OH)2/C
1 atm
MeOH
RT, 98%

NaIO4,
RuO2
Acetone, EtOAc, H2O
RT, 16 h, 90%

KHCO3
MeOH
RT, 12 h, 90%

2,6-Lutidine,
TBSOTf
CH2Cl2
0 °C, 15 min, 90%

 +
+



Ph3SnH,
AIBN
PhH
Reflux, 5.5 h, 51%
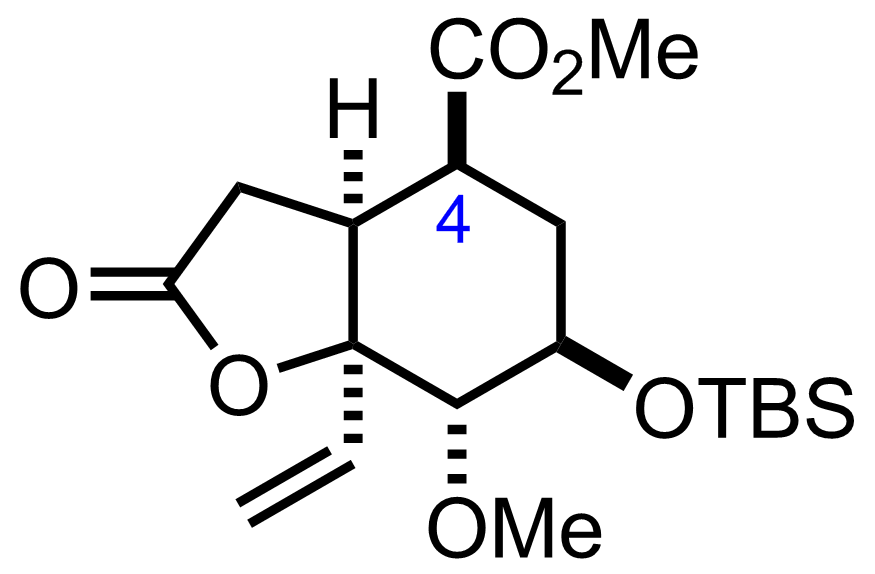
"Also isolated was the C4-epimer (22 % yield) which can be equilibrated to the desired product."

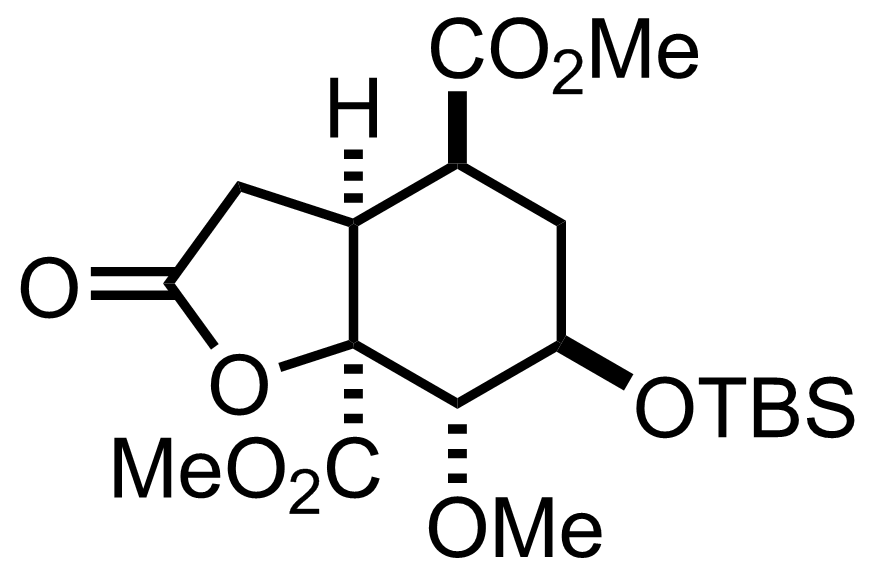
- O3
- Me2S
CH2Cl2
-78 °C to RT


CH2N2
Et2O
0 °C, 5 min, 91% (3 steps)

Sia2BH
THF
0 °C to RT, 4 h, 98%
 +
+

t-BuCO2H
PhMe
Reflux, 50 min, 53%
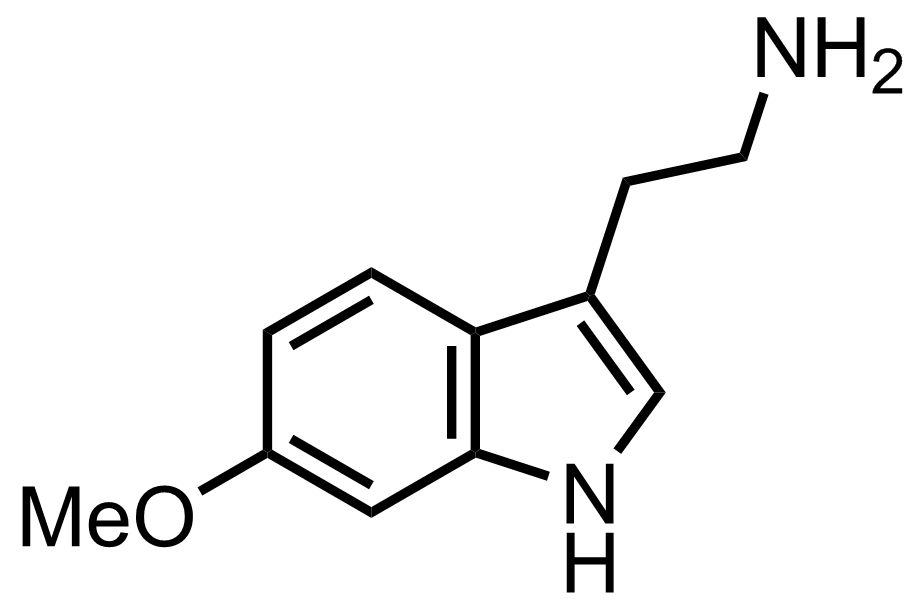
See the Pictet-Spengler Reaction
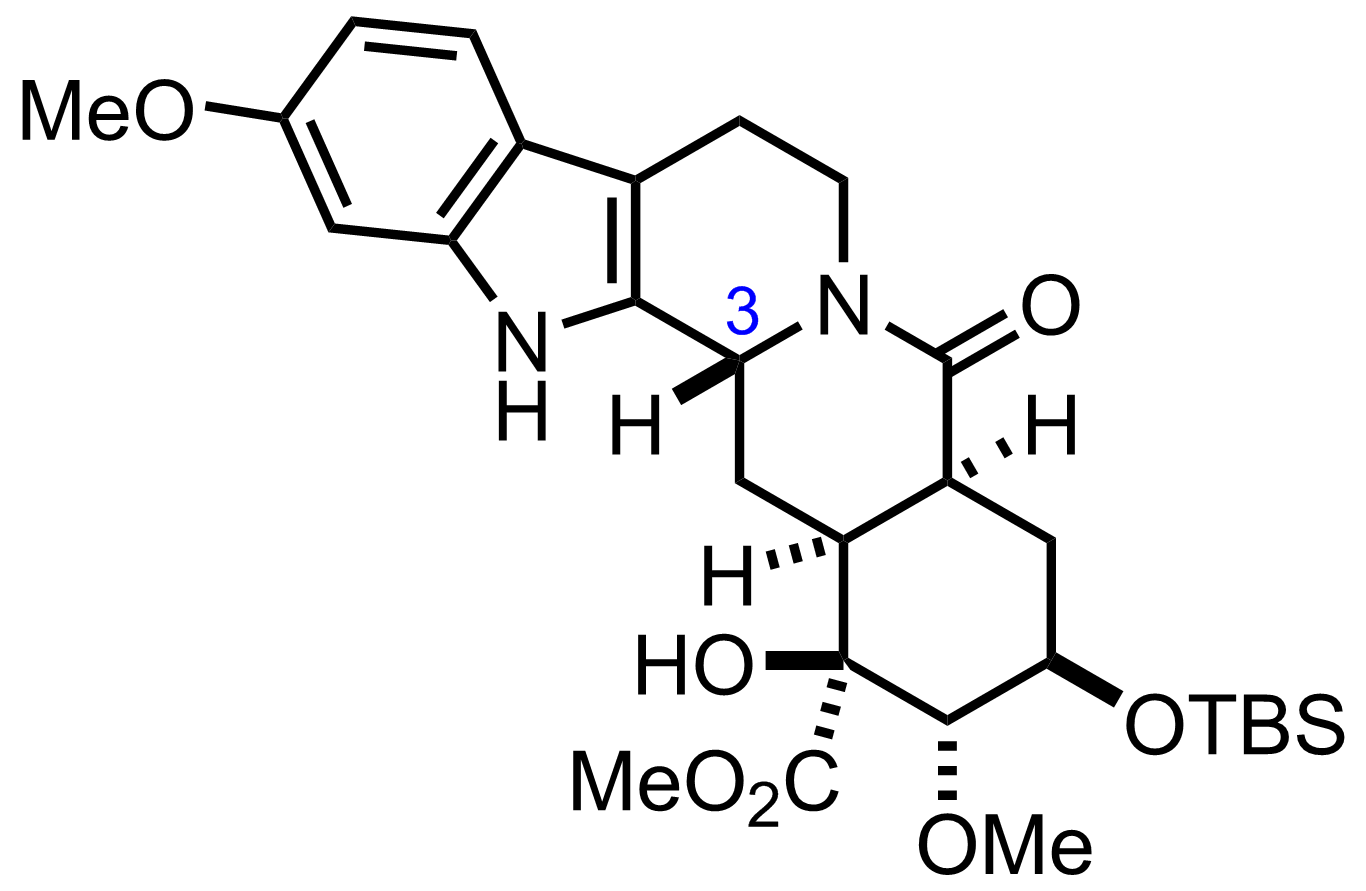
"Also isolated was the C3-epimer (37 % yield)."

2,6-Lutidine,
TMSOTf
CH2Cl2
RT, 4 h, 100%

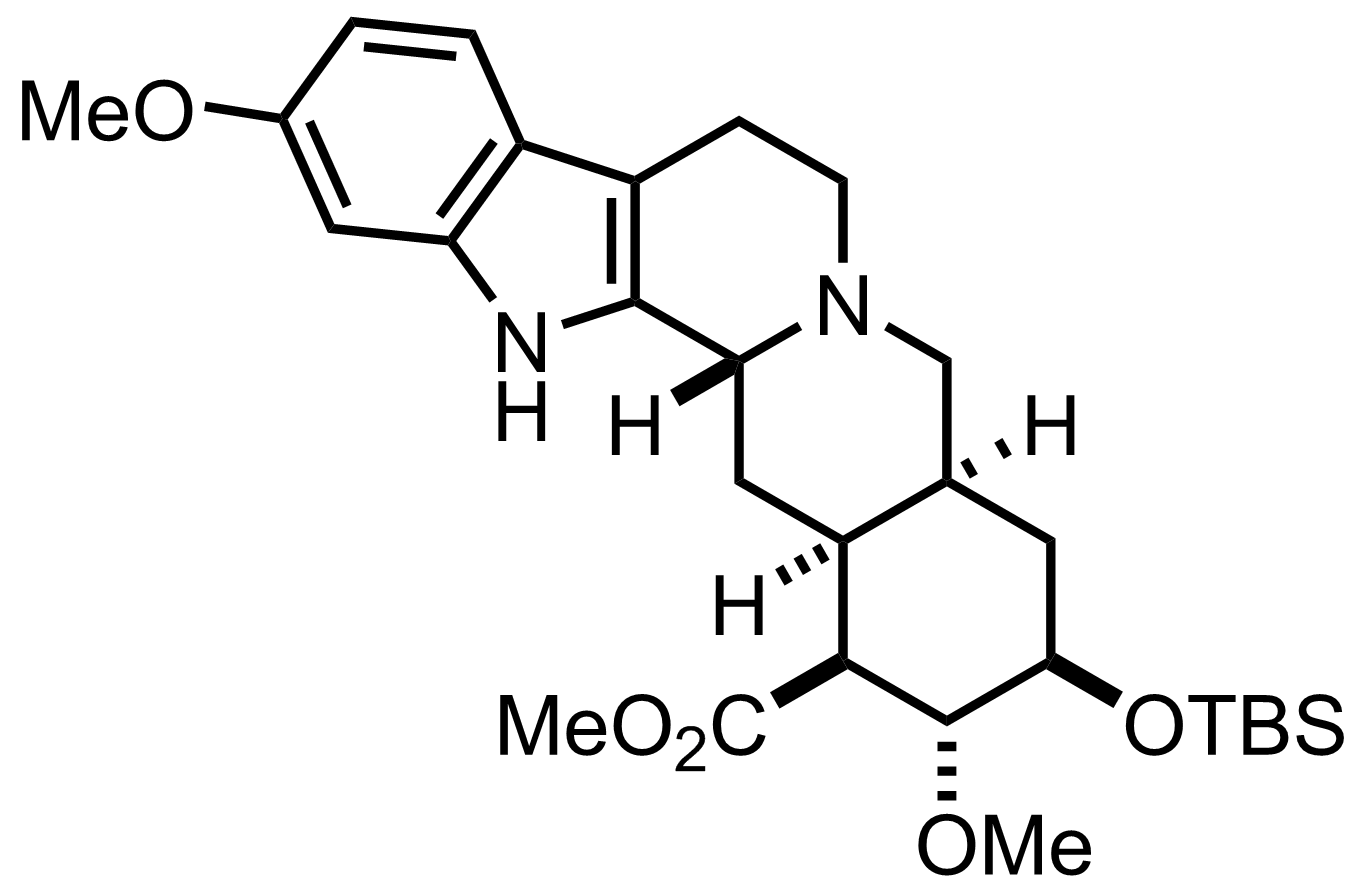
BH3.THF
THF
RT, 6 h, 84%

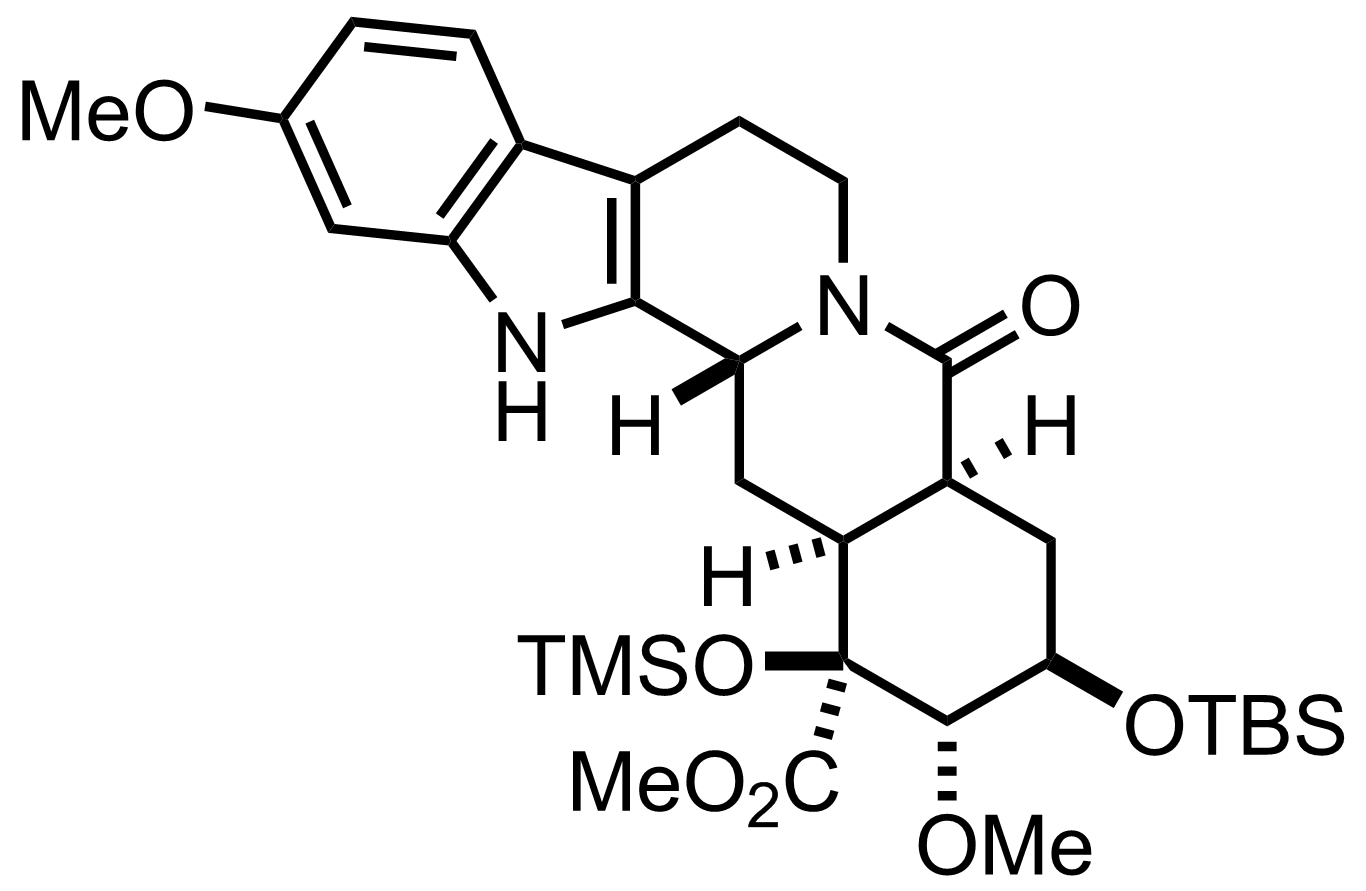
HF
H2O, MeCN
0 °C to RT, 30 min

2,6-Lutidine,
TBSOTf
CH2Cl2
0 °C, 10 min

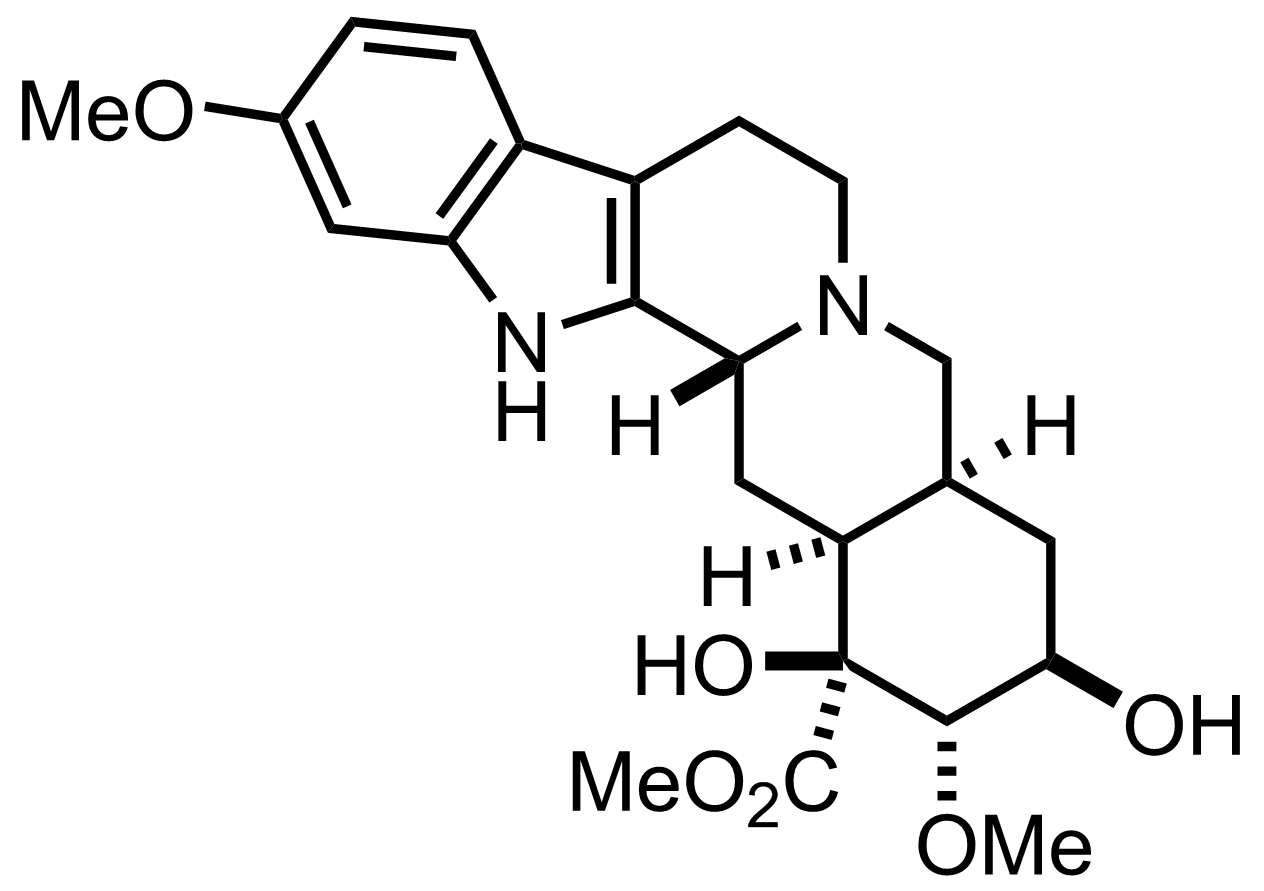
SmI2,
HMPA
Ethylene Glycol, THF
RT, 8 h, 30% (3 steps)

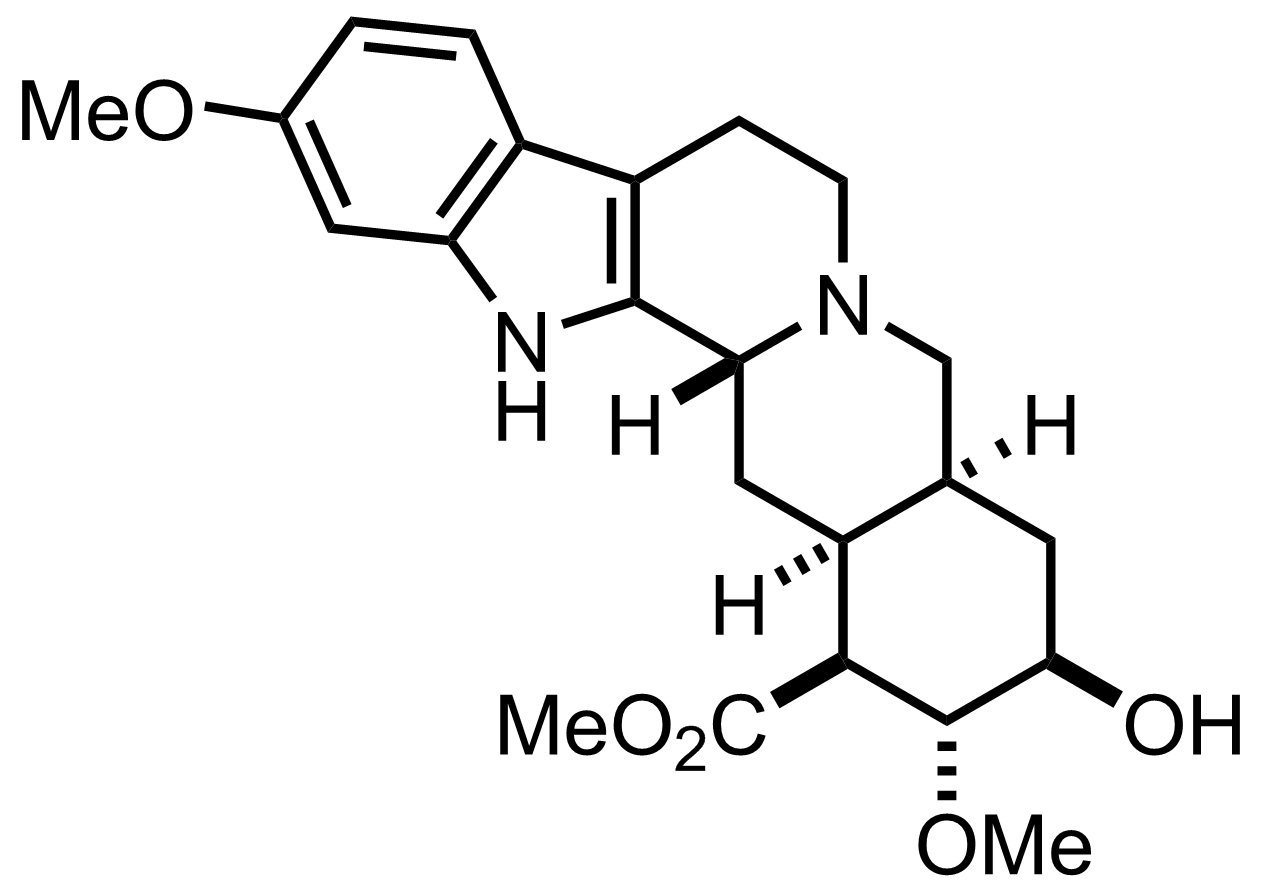
HF
H2O, MeCN
RT, 3 h
 +
+

DMAP,
Et3N
CH2Cl2
RT, ON, 80% (2 steps)

