Pinnick-Lindgren Oxidation

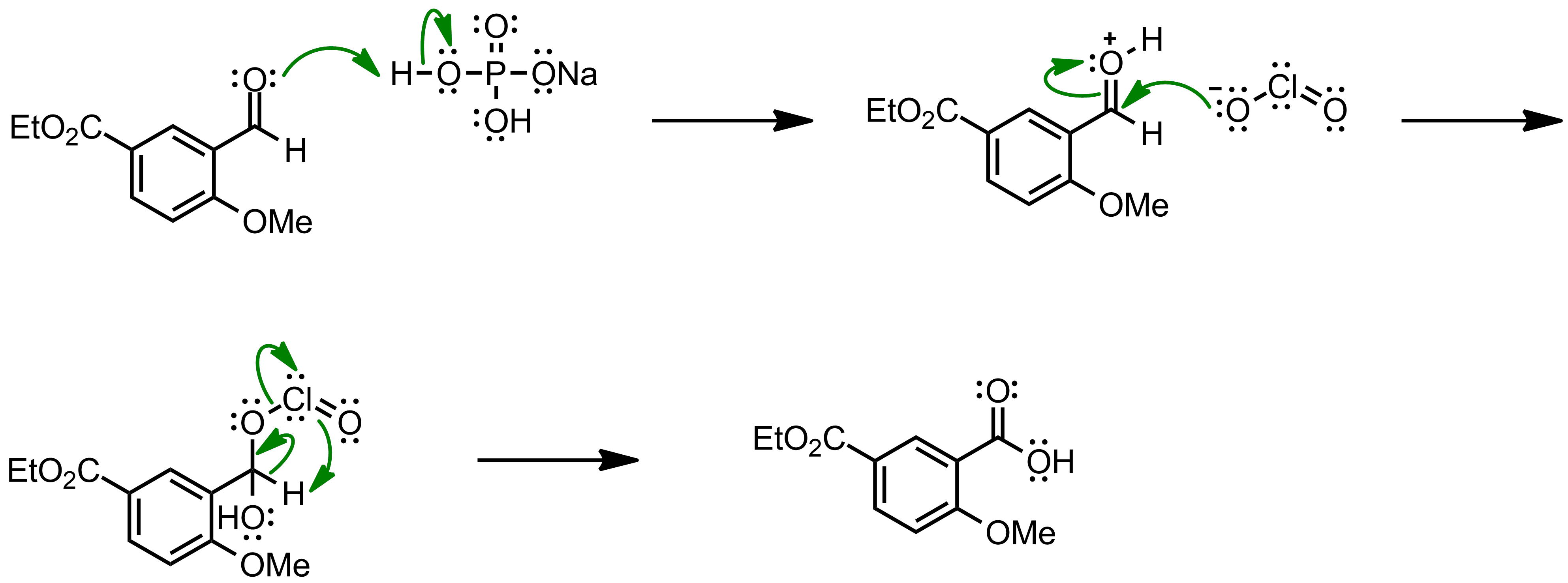
Mechanism of the Pinnick-Lindgren Oxidation

| Original publication | |
|---|---|
| Review |
|
Sample reactions
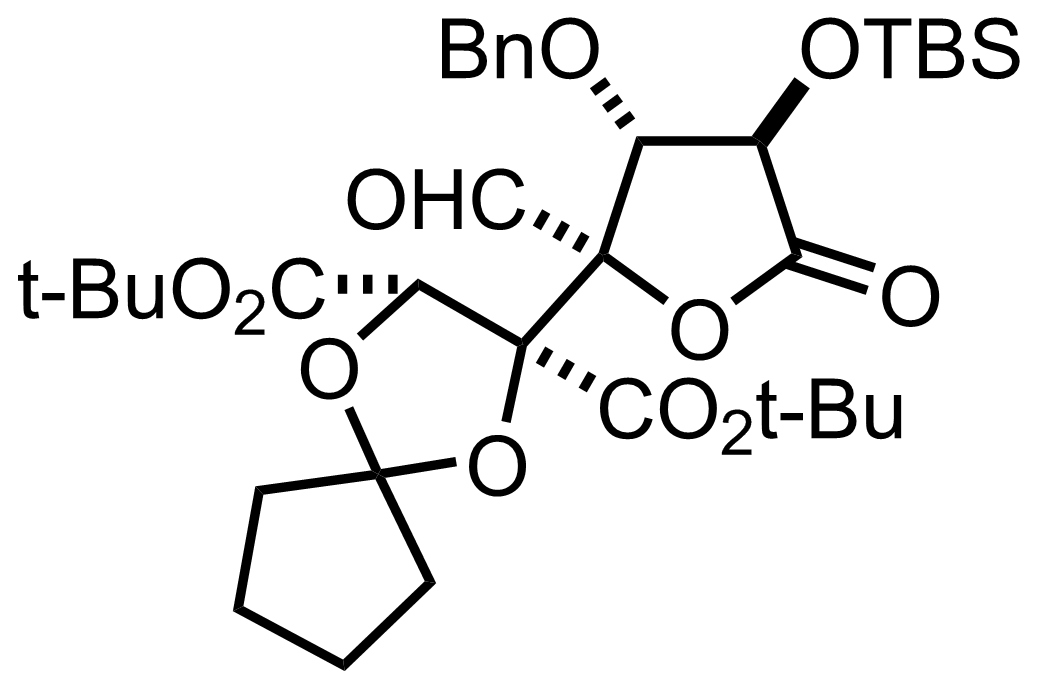
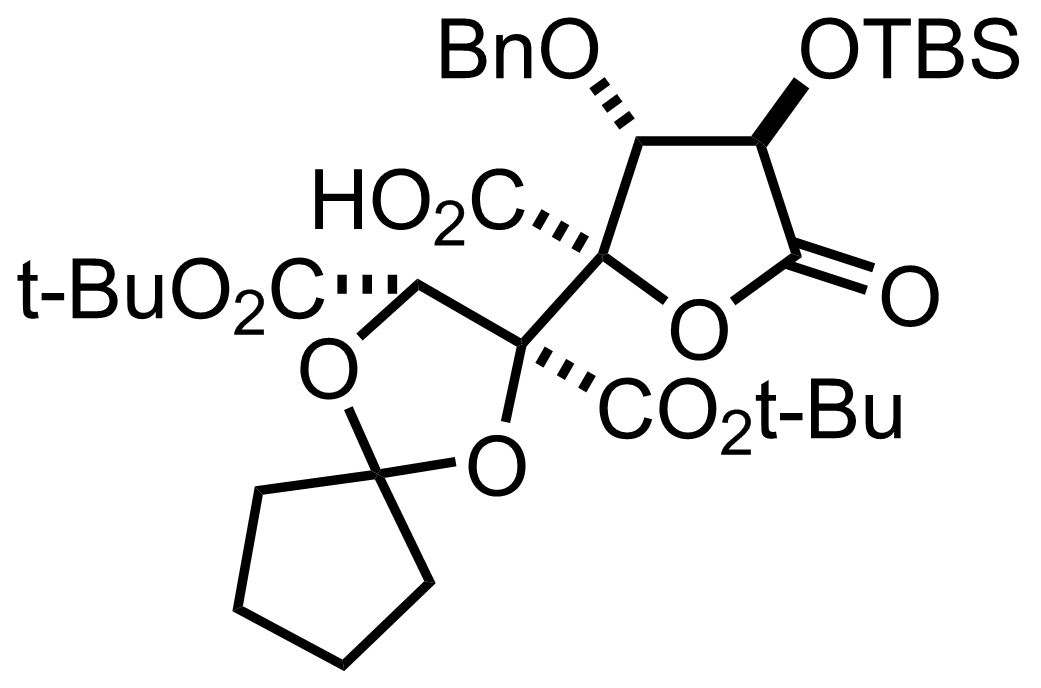
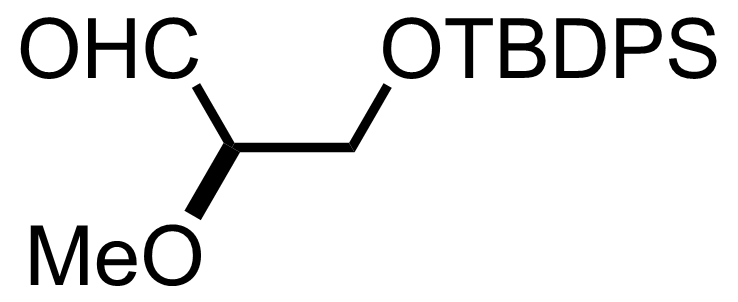
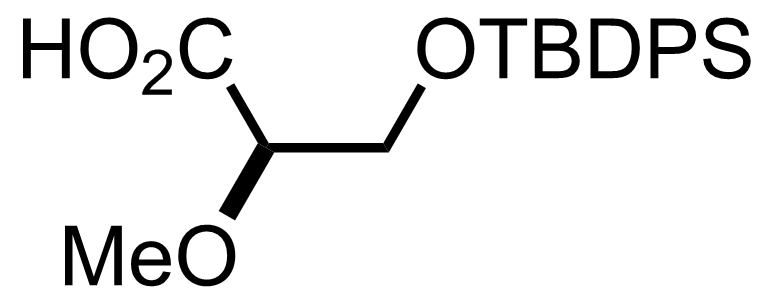
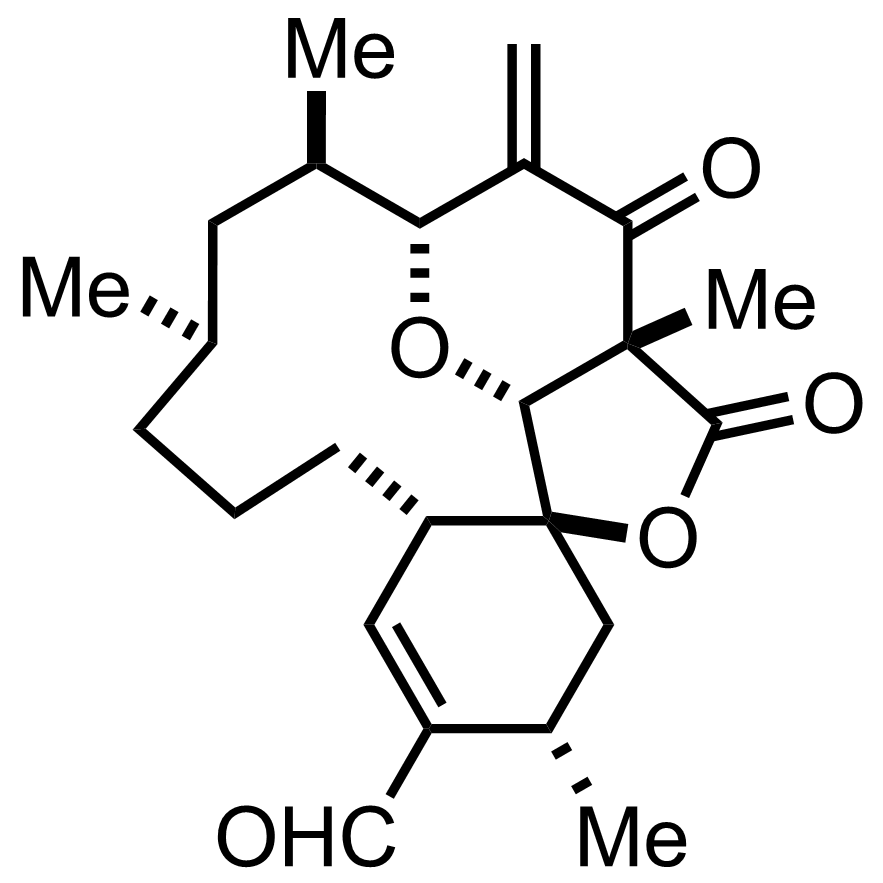
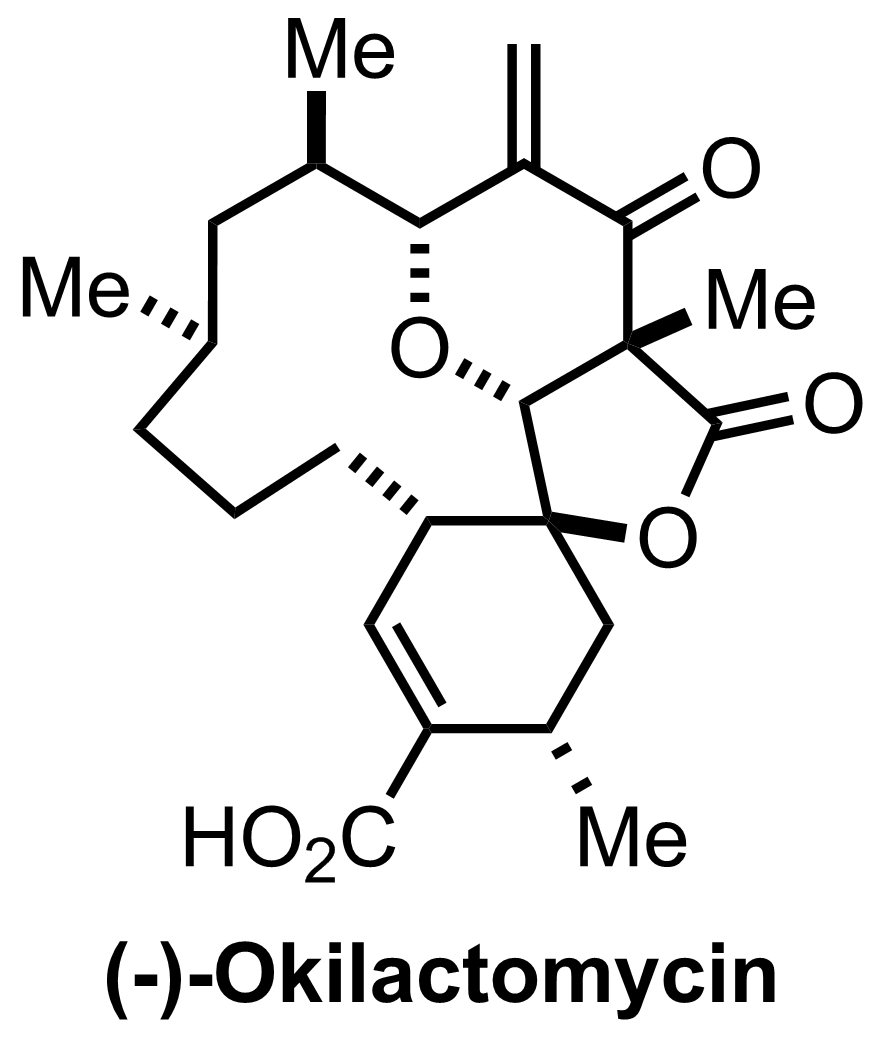

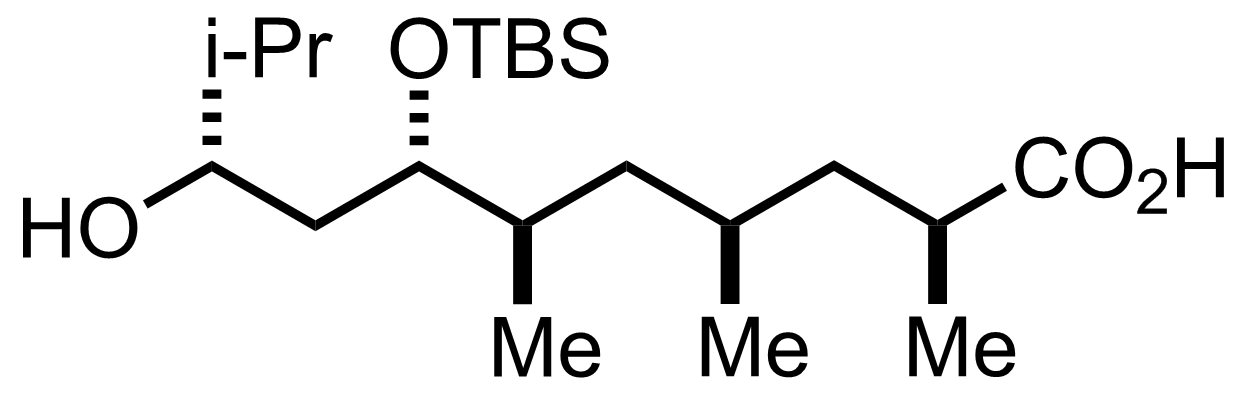
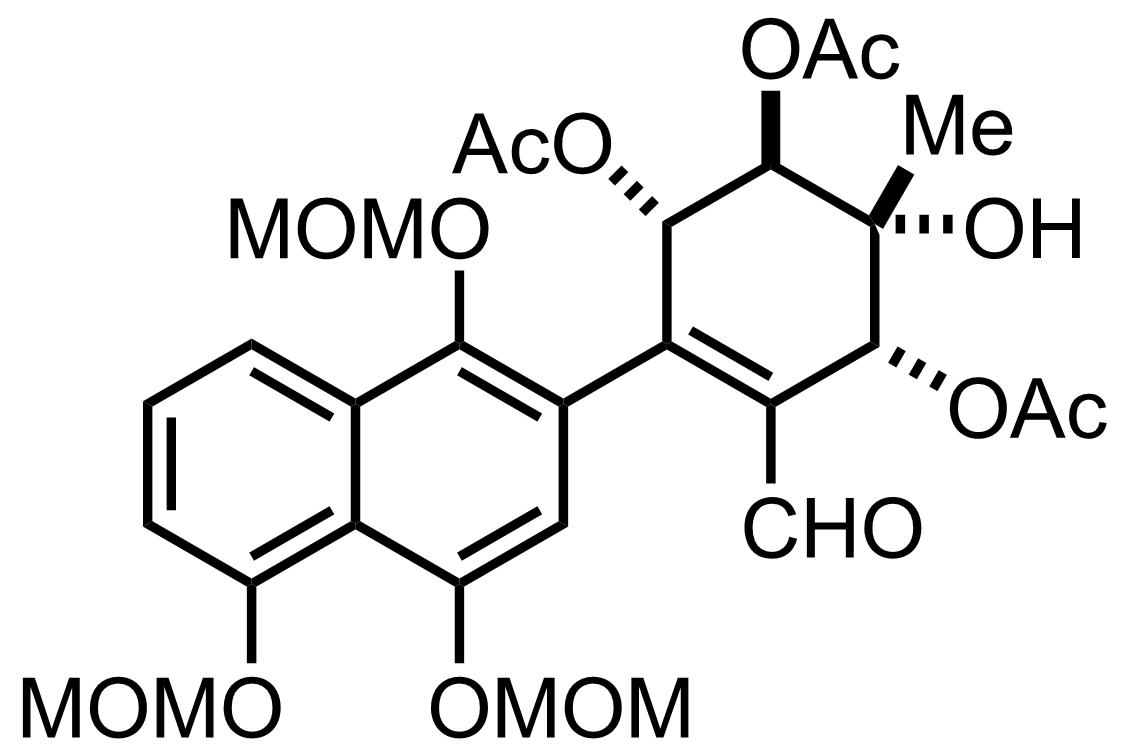
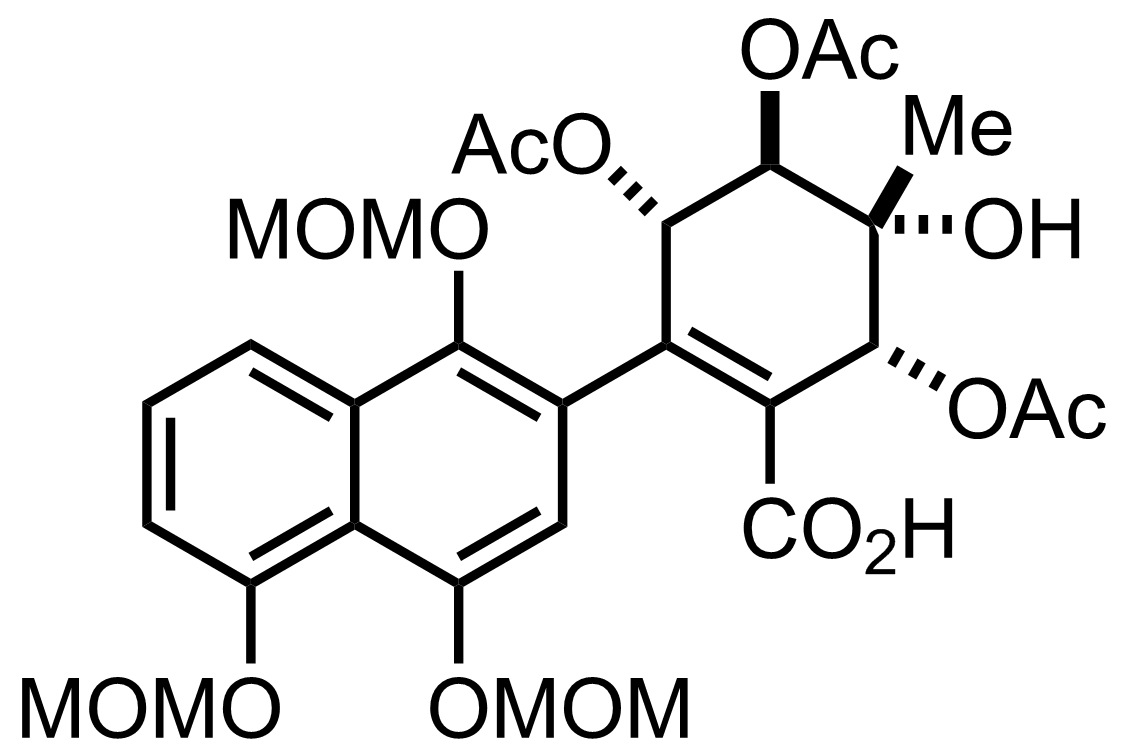


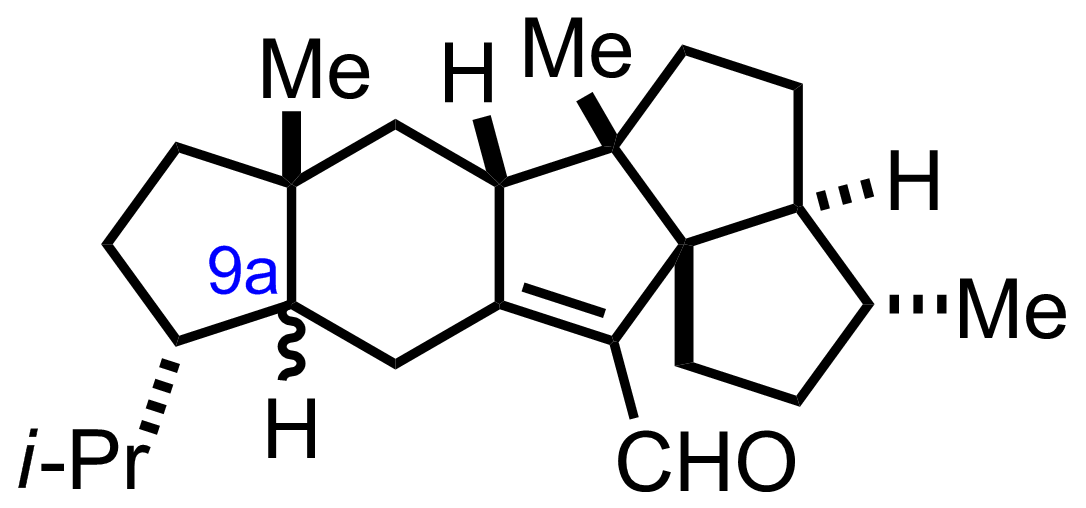




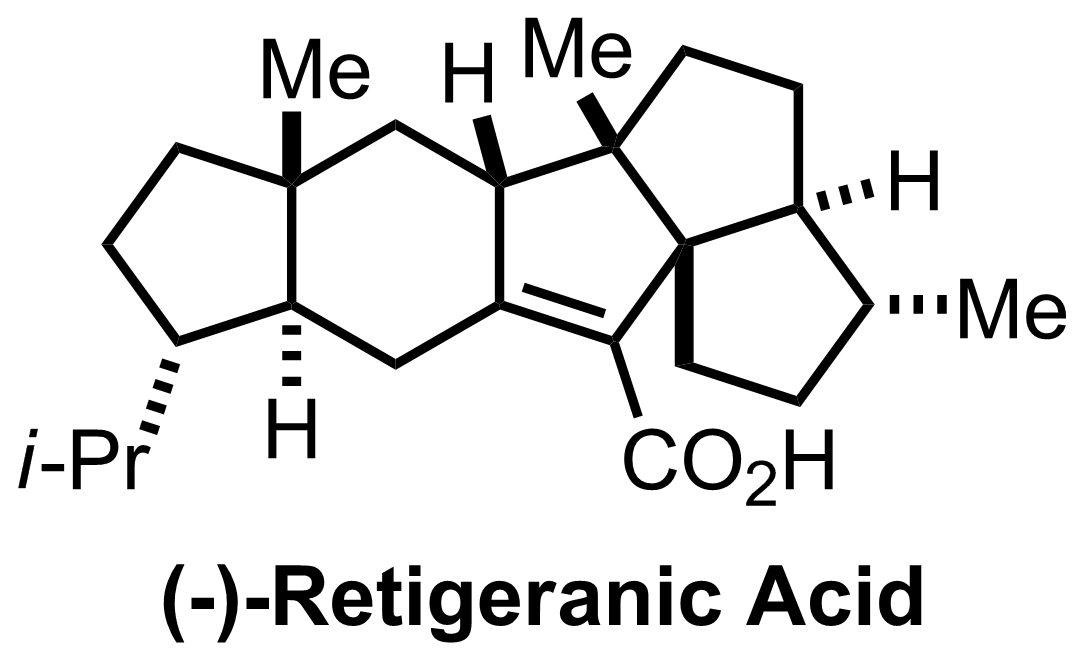
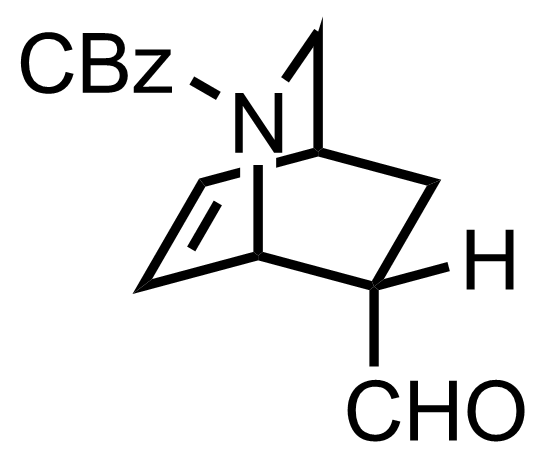

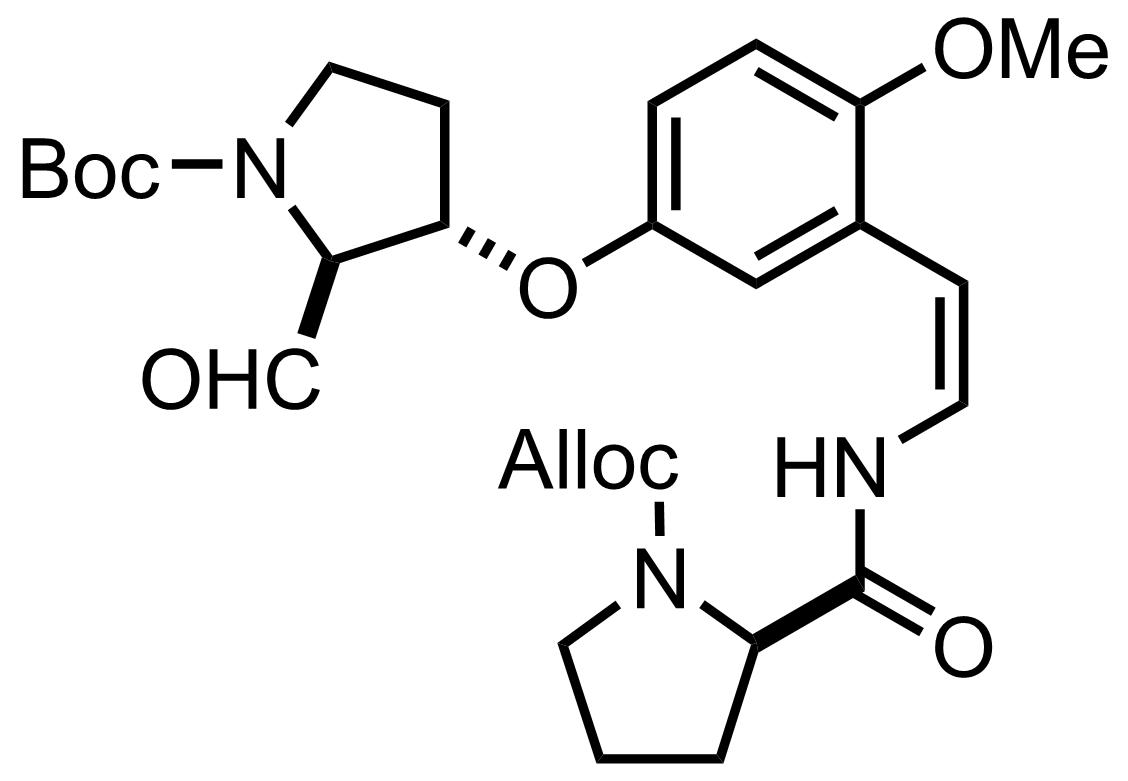
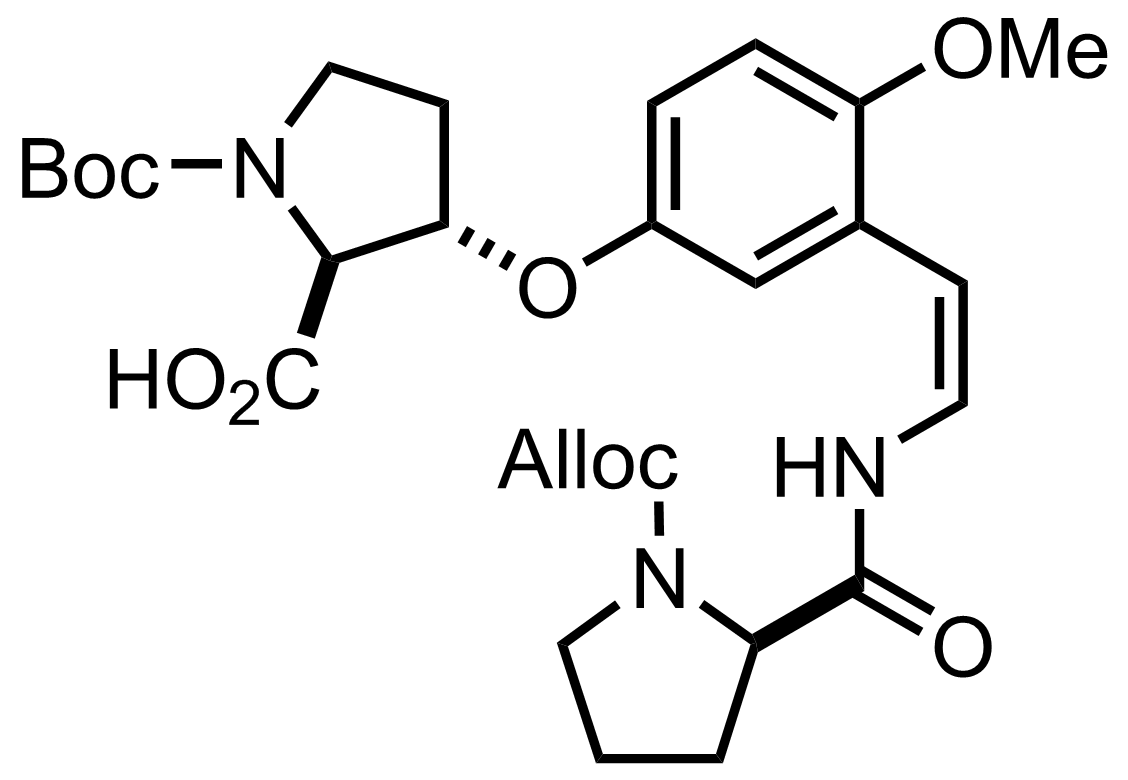
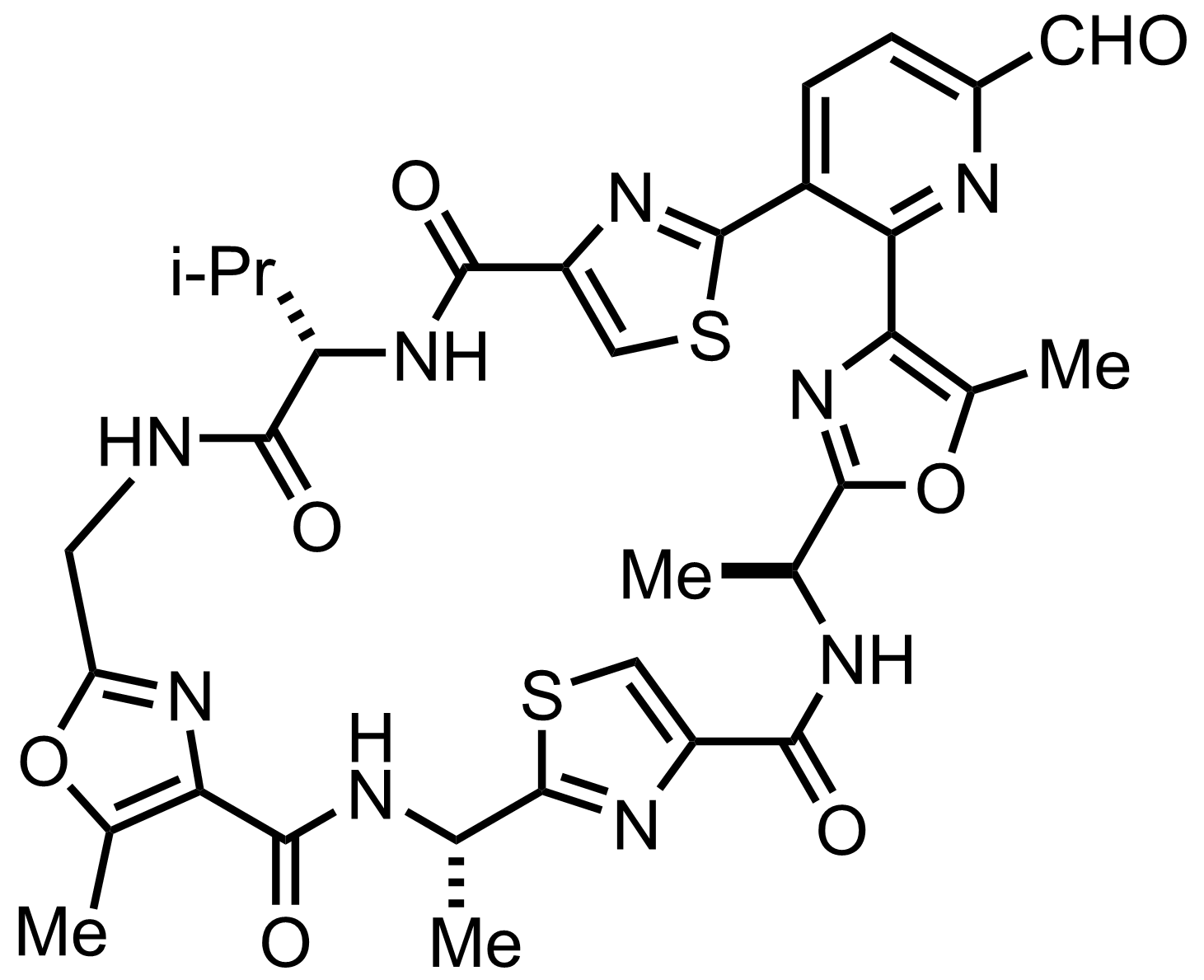
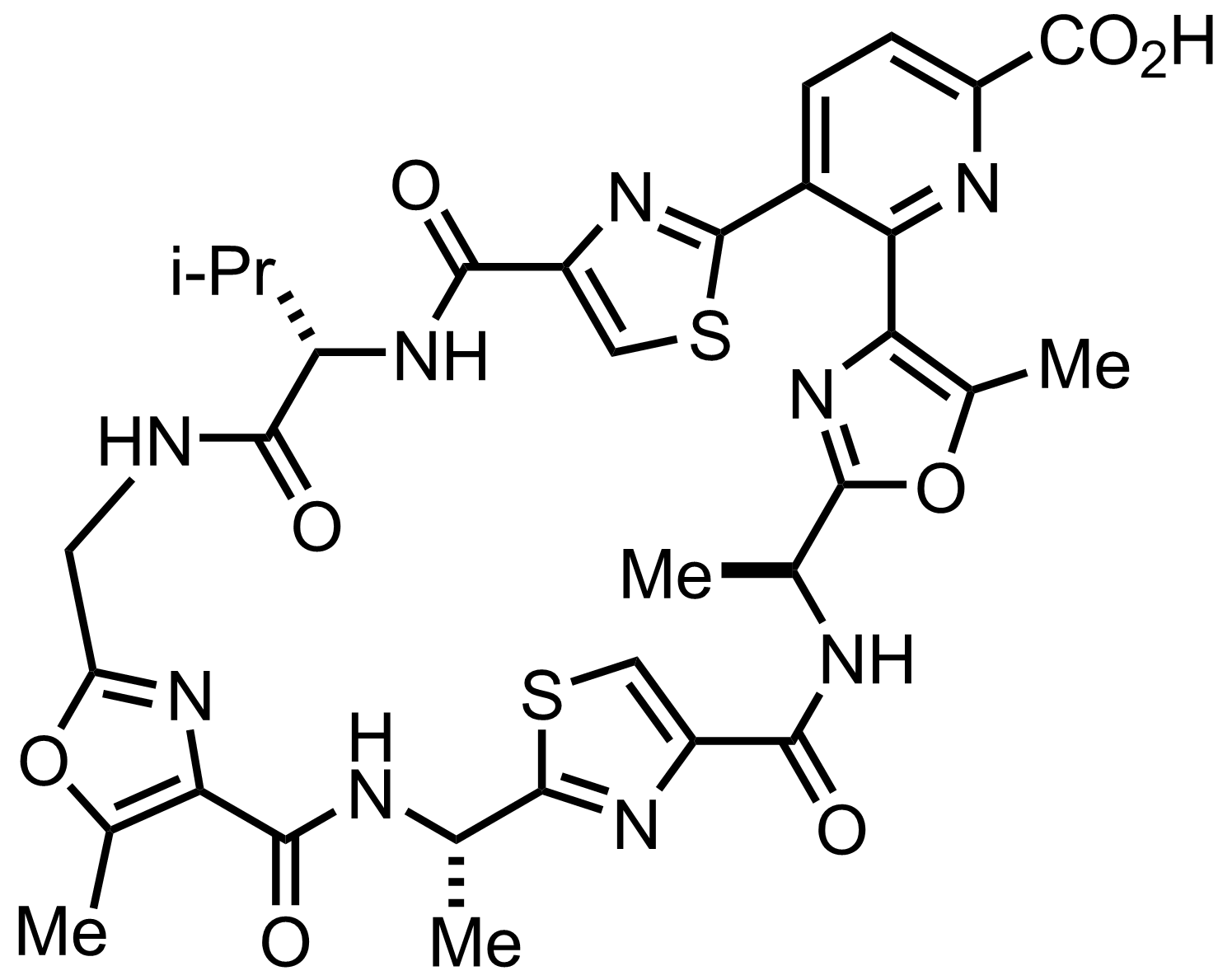
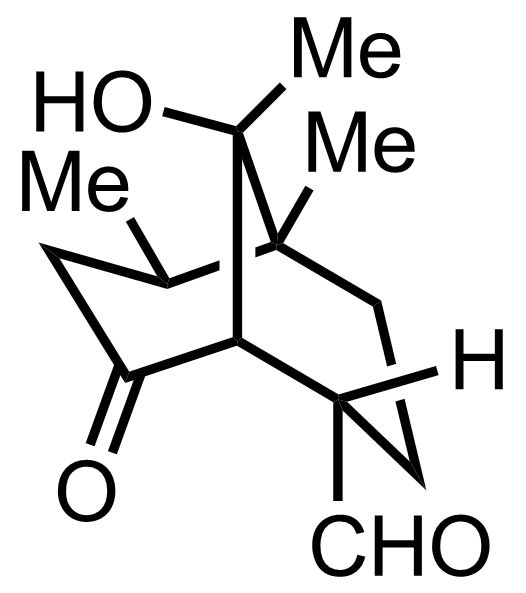

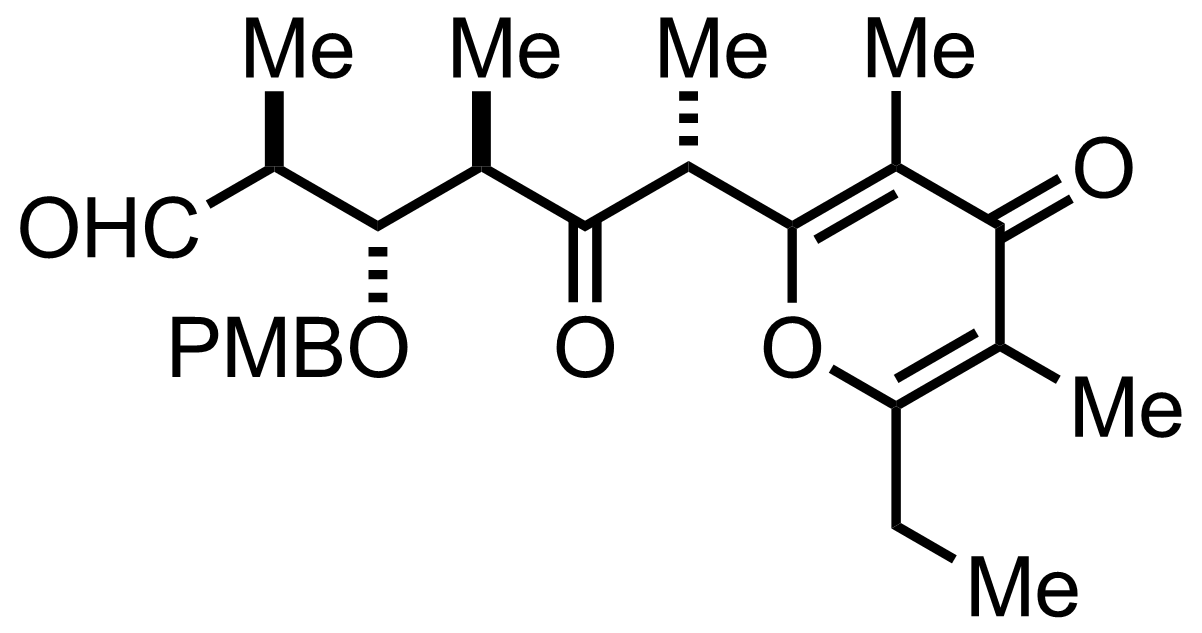
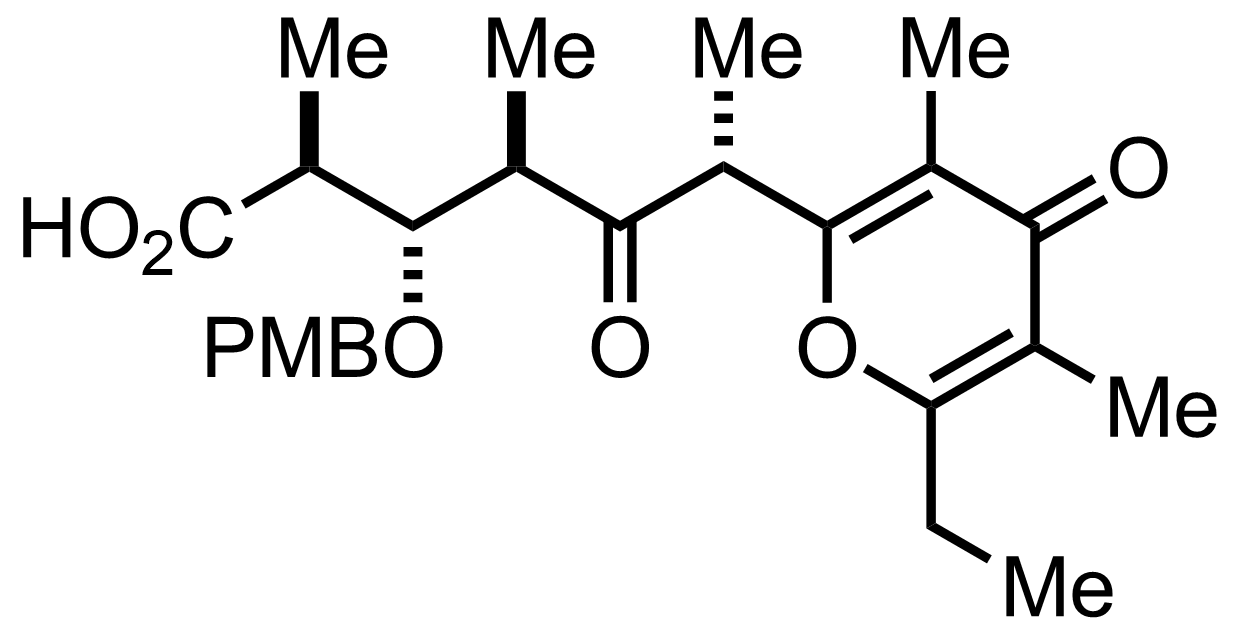



NaH2PO4, NaClO2, 2-Methyl-2-butene
t-BuOH
3.5 h


NaH2PO4, NaClO2, 2-Methyl-2-butene
t-BuOH
0 °C to RT, ON, 75% (2 steps)


NaH2PO4, NaClO2, 2-Methyl-2-butene
H2O, t-BuOH, THF
RT, 60 min, 80%


NaH2PO4, NaClO2, 2-Methyl-2-butene
H2O, t-BuOH
RT, ON


NaH2PO4, NaClO2, 2-Methyl-2-butene
H2O, t-BuOH
RT, 12 h, 88% (2 steps)


NaH2PO4, NaClO2, 2-Methyl-2-butene
H2O, t-BuOH
RT, 2 h


NaH2PO4, NaClO2, 2-Methyl-2-butene
H2O, t-BuOH
56% (2 steps)


NaH2PO4, NaClO2, 2-Methyl-2-butene
H2O, t-BuOH
RT, 30 min, 85%


NaH2PO4, NaClO2, 2-Methyl-2-butene
H2O, t-BuOH
RT, 30 min, 85%


NaH2PO4, NaClO2, 2-Methyl-2-butene
H2O, t-BuOH
0 °C to RT, 70 min


NaH2PO4, NaClO2, 2-Methyl-2-butene
MeCN, t-BuOH
0 °C to RT


NaH2PO4, NaClO2, 2-Methyl-2-butene
H2O, t-BuOH
RT, 5 h, 70%


NaH2PO4, NaClO2, 2-Methyl-2-butene
H2O, t-BuOH
0 °C, 5 min


Na2HPO4, NaClO2, 2-Methyl-2-butene
H2O, t-BuOH
RT, 1 h, 96% (2 steps)


NaH2PO4, NaClO2, 2-Methyl-2-butene
H2O, t-BuOH
0 °C, 70 min, 99%

